Topics
12/30/2025
Biological Responses Driving Weight Rebound After Weight Loss
<Summary>
When individuals lose weight through calorie restriction, adaptive responses involving coordinated changes in metabolism, neuroendocrine function, autonomic regulation, and behavior are triggered, ultimately promoting weight rebound. These responses may help explain why calorie-restricted diets frequently fail to produce lasting weight loss.
(1) Metabolic adaptation
Weight loss induced by calorie restriction leads to a significant reduction in resting energy expenditure beyond what would be predicted from changes in body composition. This metabolic adaptation occurs in both formerly obese individuals and naturally lean individuals who have lost weight, creating conditions that favor weight rebound.
(2) Endocrine function
A wide range of hormones secreted from the gastrointestinal tract and adipose tissue—such as leptin, ghrelin, peptide YY, and cholecystokinin—play essential roles in regulating appetite, food intake, and energy expenditure. A calorie-restricted diet simultaneously decreases satiety and increases hunger, which can promote overeating.
(3) Food reward and addiction-like processes
When we eat palatable foods, neurotransmitters such as dopamine are released, activating reward-related neural circuits. The desire to experience this pleasure again motivates the next eating episode. Calorie restriction and fasting can heighten the reward value of food, especially energy-dense, highly palatable foods.
(4) Inhibitory system and binge eating
Short-term dieting success can be attributed to enhanced inhibitory neural responses that temporarily suppress the desire to eat. However, as dietary restrictions continue, activation of reward-related brain regions may begin to override inhibitory control, making it increasingly difficult to resist cravings for palatable foods.
(5) Adipose cellularity
Weight loss reduces the size of adipocytes (fat cells), but their number generally remains unchanged. However, some researchers have pointed out that the possibility of new fat cells forming (hyperplasia) during weight rebound cannot be completely ruled out. If hyperplasia does occur, these fat cells could enlarge again, potentially promoting further expansion of overall adipose tissue.
(6) Intestinal starvation
Unlike the situations described in (1)–(5), intestinal starvation does not arise from a marked deficiency in energy.
Conclusion
Some researchers argue that the biological forces driving weight rebound after weight loss are extremely powerful and difficult to overcome. In my opinion, rather than trying to fight these responses, it is more important to avoid triggering them strongly in the first place.
Specifically, instead of enduring prolonged hunger, it is helpful to consume more nutrient-dense, minimally processed foods while adjusting caloric intake. Doing so helps sustain satiety and reduce hunger—key factors in supporting long-term weight management.
【Full text】
-
Contents
- Various mechanisms that promote weight rebound
(1)Metabolic adaptation
(2) Endocrine Function
(3) Food Reward and Addiction-Like Processes
(4) Inhibitory system and binge eating
(5) Adipose cellularity
(6) Intestinal starvation - Conclusion
- Various mechanisms that promote weight rebound
<Introduction>
The prescription for people with obesity to ”eat less and exercise more”remains a widely used approach to weight management, despite its well-documented failures[1]. It has been suggested that most of the weight lost through dieting is regained over the long term[2].
According to research in genetics, epidemiology, and physiology, body fat and body weight are known to be tightly regulated. When a person attempts to maintain weight loss, adaptive responses involving coordinated changes in metabolic, neuroendocrine, autonomic, and behavioral functions are triggered, acting to oppose the maintenance of the reduced weight[4].
In this post, I’d like to take a brief look at these biological mechanisms that may drive weight rebound and even promote further weight gain after dieting. I will also explain how my theory of intestinal starvation differs from these mechanisms.
【Related Articles】 The Spread of Dieting May Be Fueling the Rise in Obesity
1.Various mechanisms that promote weight rebound
(1) Metabolic adaptation
Energy restriction is associated with a reduction in resting energy expenditure (REE)[5].
Many studies have reported that behavioral weight loss leads to a greater decrease in both resting and total energy expenditure than would be predicted based on changes in body composition and the thermic effects of food[4,6].
This phenomenon, known as adaptive thermogenesis (AT) or metabolic adaptation, creates conditions that favor regaining lost weight[7].
Metabolic adaptation can be interpreted teleologically as the body's survival response: when the body perceives a state of starvation, it reduces the energy costs of living in an attempt to prolong life. Interestingly, this response also appears to occur in individuals with obesity and does not seem to be diminished by the quantity of energy stored as body fat[7,8].

(Author: rawpixel.com / Source: Freepik)
However, regarding the timing of its onset, there is inconsistent evidence[9]. Some studies have detected adaptive thermogenesis (AT) within a week of energy restriction, which has been associated with rapid declines in insulin secretion, depletion of glycogen stores, and loss of intra-and extra cellular fluid[10].
In contrast, a growing body of evidence suggests that underfeeding-associated AT takes weeks to develop[11], primarily in association with reduced leptin secretion resulting from the reduction of stored fat[9,12].
Although the persistence of AT also remains a subject of debate[7], some studies indicate that this metabolic adaptation may continue for years even after energy balance has been reestablished at a lower weight[13].
(2) Endocrine function
A number of hormones secreted from the gastrointestinal tract and adipose tissue are known to play key roles in regulating appetite, food intake, energy expenditure, and body weight[14,15].
Leptin is a hormone secreted by fat cells that helps regulate body weight by suppressing appetite through stimulation of the satiety center and by increasing energy expenditure. High leptin levels are interpreted by the brain that energy reserves are high, whereas low leptin levels indicate that energy reserves are low[16].
It has been shown that leptin levels drop within 24 hours of energy restriction[17]. Interestingly, many studies have reported a greater reduction in leptin levels than would be expected for given losses of adipose tissue[18,19].
It has been suggested that the primary role of leptin may be the prevention of starvation, rather than weight regulation per se[15,20]. When leptin levels fall below a certain threshold—the point at which specific physiological responses are triggered(*1)—starvation defense mechanisms are activated, even if substantial fat stores remain[17]. This leads to a reduction in metabolic rate and physical activity, as well as an increase in hunger[21,22].
(* 1) It has been suggested that this threshold rises as fat mass increases[17].
Furthermore, in individuals who have lost weight, an increase in the appetite-stimulating hormone ghrelin, along with decreases in the post-meal satiety signals peptide YY (PYY) and cholecystokinin (CCK), has been observed[23].
As a result, behavioral weight loss can simultaneously induce a decrease in satiety and an increase in hunger, potentially promoting overeating[15].
(3) Food reward and addiction-like processes
Food reward refers to the brain’s mechanism that generates pleasure and satisfaction from eating, as well as the motivation or desire to eat again. This process involves activation of the brain’s reward circuitry, where neurotransmitters such as dopamine are released, leading to feelings of well-being and increased appetite.

(Author: rawpixel.com / Source: Freepik)
The regulation of food intake is influenced by a close interaction between homeostatic and non-homeostatic (hedonic) factors.
The former is related to nutritional needs, monitoring available energy in the blood and fat stores to maintain energy balance. The latter, in contrast, is largely associated with the brain’s reward system[24,25].
Although the mechanisms that determine how much we eat are largely homeostatic, reward-related signals can easily override these normal satiety signals that help maintain a stable body weight, potentially leading to overeating[25,26].
Modern neuroimaging studies using fMRI have shown that both nutritional status (e.g., hunger vs. satiety) and different food stimuli (e.g., high vs. low calorie, appetizing vs. bland foods) can alter activity in the brain’s reward circuitry[27,28,29].
Recent studies in healthy individuals indicate that short-or long-term caloric restriction, as well as fasting, may increase the reward value of food—especially for high-calorie, palatable items[27,30].
These findings may help explain why calorie-restricted diets for weight loss often fail in the long term[28,30].
<Food Addiction and Its Differences from Drug Addiction>
While drugs and food share certain characteristics, they also differ in qualitative and quantitative ways.
Drugs of abuse, such as cocaine, act directly on the brain’s dopamine circuitry, whereas food influences the same circuits more indirectly. Signals from taste and smell, nutrient sensors in the digestive tract[31], and hormones released during digestion and absorption of ingested food all communicate with the brain and activate the dopamine system[25].

Although it remains debated whether specific food components such as sugar, sweeteners, salt, or fat can prompt addictive processes[25], highly palatable and calorie-dense foods—such as chocolate, ice cream, cookies, and salty snacks—can serve as powerful rewards.
In today’s stress-filled society, these foods provide pleasure and comfort, leading some researchers to draw parallels between “food addiction” and drug addiction[32,33].
(4) Inhibitory system and binge eating
Food intake is primarily regulated by three interactive neural systems: the homeostatic, reward-related, and inhibitory systems[15].
The inhibitory system—mainly involving the brain region responsible for self-control and decision-making—helps regulate eating behavior and inhibit excess food intake[34].
<Cognitive control of food reward>
In humans, the urge to seek and consume palatable foods can be moderated by cognition, specifically executive functions. One of the central dilemmas in daily life is balancing one’s internal goals (e.g., cutting back on sweets to maintain health and weight control) against the immediate reward of eating tempting foods. This conflict is particularly challenging when highly desirable foods, like donuts or pizza, are readily available[25].

(Source: Freepik)
The short-term success in dieting suggests that an increase in inhibitory neural responses can temporarily override the neurobiological drive to consume highly palatable high-calorie foods[35].
However, recent evidence indicates that reward-related neural signaling is activated in conjunction with inhibitory signaling[36].
In simple terms, as dietary restriction continues, it may become increasingly difficult to resist the urge to eat appetizing, high-reward foods.
Prospective studies in young individuals, as well as animal experiments in rodents, suggest that severe caloric restriction, characterized by 24-hour fasting or fat-free diets, may increase the risk of developing binge eating and bulimia in the future[37,38].
(5) Adipose cellularity
Weight loss dieting may reduce the size but not the number of fat cells[39]. It remains unclear whether hyperplasia (an increase in adipocyte number) contributes to weight rebound in weight-suppressed individuals[15]. However, in a study with obese rats, adipocyte hyperplasia has been observed following refeeding after fasting[40].
In humans, a similar possibility has been suggested[15].
Normally, when energy availability is low, triglycerides stored in fat tissue are broken down to supply energy to cells.
However, the rate of lipolysis (fat breakdown) appears to be related to adipocyte size and cellular surface area[41]—meaning that as fat cells shrink, their rate of lipolysis tends to decline.
If size-reduced adipocytes are functionally modified to break down less and store more fat, these cells may become enlarged, potentially promoting the overall expansion of adipose tissue[15,42].

(Author: brgfx / Source: Freepik)
(6) Intestinal starvation
The reactions described in section 1 to 5 are thought to represent a series of anti-starvation or anti-weight loss mechanisms(* 2) triggered by glycogen depletion or a significant decrease in stored body fat[15].
In contrast, intestinal starvation does not result from a depletion of energy. While it can occur under strict dietary restrictions aimed at weight loss—such as skipping meals or eating only very small portions—it may also be triggered by more casual dieting, or lifestyle habits not directly related to dieting, such as skipping breakfast, eating light lunches, having late dinners, or eating two meals a day.
【Related article】
Defining "Intestinal Starvation": Its Relevance to the Multifactorial Model of Obesity
I also believe that when intestinal starvation is induced, it leads to overall weight gain, suggesting an increase in body’s set-point weight. This increase likely involves not only body fat but also lean tissue such as muscle mass. Therefore, it may differ from weight gain mechanisms characterized by abnormal increases in abdominal and total body fat.
(* 2) Some researchers prefer the term "anti-weight loss" mechanism rather than anti-starvation, because these responses operate despite the presence of adequate energy stores[15].
2. Conclusion
Although a direct causal relationship between the five mechanisms (section 1-5) discussed here and weight rebound has not yet been proven[15], many people who have experienced rebounding after dieting may find these explanations relatable.
Some researchers point out that “the biological forces resisting weight loss and driving weight rebound are so powerful that most individuals attempting to lose weight through behavioral interventions are unlikely to overcome them.” At the same time, they emphasize the need to develop new strategies that can weaken these biological mechanisms in order to achieve long-term weight loss[15].
In my opinion, rather than trying to overcome these powerful biological forces, what’s truly important is to avoid triggering the anti-starvation (or anti-weight loss) mechanisms in the first place.
Currently, obesity is widely believed to result from excess caloric intake and/or lack of physical activity. Consequently, the common advice is to “eat less and exercise more.” However, many people try to reduce calories by eating light meals or very small portions (e.g., a simple sandwich, a simple burger) and endure extended periods of hunger. As the findings on food reward and inhibitory systems indicate, this approach clearly ignores the body’s natural biological mechanisms.
I would rather recommend the following approach:
• Mainly reduce refined carbohydrates and adjust total caloric intake—but avoid extreme restrictions.
• Increase other foods, such as fiber-rich vegetables, seaweed, dairy products, minimally processed meats and fish, and nuts. In particular, emphasize foods that are harder to digest or take longer to break down (* 2).
(* 2) Even high-calorie foods like oils and nuts can be appropriate depending on how they’re consumed.
By maintaining this dietary approach, the signal that "there is sufficient food available" may be transmitted through the gut-brain axis. Sustaining satiety and reducing hunger is key.
Moreover, nutrient-sensing systems in the digestive tract and other parts of the body have also been indicated to contribute to the generation of food reward during and after a meal[43]. By chewing slowly and savoring each bite, you can gain not only the immediate reward from taste buds but also a longer-lasting sense of satisfaction that extends well beyond the end of the meal[44].
The current obesity epidemic is often described as a mismatch between our modern, food-abundant environment and biological response patterns that evolved under food-scarce conditions[44,45].
From this perspective, I believe that in an environment where palatable food is readily available, even extended periods of hunger in daily life may actually promote long-term increases in body fat depending on how we combine foods.
<References>
[1] Bacon L, Aphramor L. Weight science: evaluating the evidence for a paradigm shift. Nutr J. 2011 Jan 24;10:9.
[2]National Institutes of Health Technology Assessment Conference Panel (1993) Methods for voluntary weight loss and control. Ann Intern Med 119, 764–770.
[3] Deleted
[4] Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond). 2010 Oct;34 Suppl 1(0 1):S47-55.
[5]Jiménez Jaime T et al. Effect of calorie restriction on energy expenditure in overweight and obese adult women. Nutr Hosp. 2015 Jun 1;31(6):2428-36.
[6]Johannsen DL et al. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. 2012 Jul;97(7):2489-96.
[7]Hall KD, Guo J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology. 2017 May;152(7):1718-1727.e3.
[8]Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995 Mar 9;332(10):621-8.
[9]Egan AM, Collins AL. Dynamic changes in energy expenditure in response to underfeeding: a review. Proc Nutr Soc. 2022 May;81(2):199-212.
[10]Heinitz S et al. Early adaptive thermogenesis is a determinant of weight loss after six weeks of caloric restriction in overweight subjects. Metabolism. 2020 Sep;110:154303.
[11] Dulloo AG, Seydoux J, Jacquet J. Adaptive thermogenesis and uncoupling proteins: a reappraisal of their roles in fat metabolism and energy balance. Physiol Behav. 2004 Dec 30;83(4):587-602.
[12]Müller MJ, Enderle J, Bosy-Westphal A. Changes in Energy Expenditure with Weight Gain and Weight Loss in Humans. Curr Obes Rep. 2016 Dec;5(4):413-423.
[13]Fothergill E et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring). 2016 Aug;24(8):1612-9.
[14]Schwartz MW et al. Central nervous system control of food intake. Nature. 2000 Apr 6;404(6778):661-71.
[15]Ochner CN et al. Biological mechanisms that promote weight regain following weight loss in obese humans. Physiol Behav. 2013 Aug 15;120:106-13.
[16]Hebebrand J et al. The role of hypoleptinemia in the psychological and behavioral adaptation to starvation: Implications for anorexia nervosa. Neurosci Biobehav Rev. 2022 Oct;141:104807.
[17]Leibel RL. The role of leptin in the control of body weight. Nutr Rev. 2002 Oct;60(10 Pt 2):S15-9; discussion S68-84, 85-7.
[18]Löfgren P et al. Long-term prospective and controlled studies demonstrate adipose tissue hypercellularity and relative leptin deficiency in the postobese state. J Clin Endocrinol Metab. 2005 Nov;90(11):6207-13.
[19]Rosenbaum M et al. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab. 1997 Nov;82(11):3647-54.
[20]Ahima RS et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996 Jul 18;382(6588):250-2.
[21] Rosenbaum M et al. Energy intake in weight-reduced humans. Brain Res. 2010 Sep 2;1350:95-102.
[22]Kissileff HR et al. Leptin reverses declines in satiation in weight-reduced obese humans. Am J Clin Nutr. 2012 Feb;95(2):309-17.
[23] Sumithran P et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011 Oct 27;365(17):1597-604.
[24]Chaptini L, Peikin S. Neuroendocrine regulation of food intake. Curr Opin Gastroenterol. 2008 Mar;24(2):223-9.
[25]Alonso-Alonso M et al. Food reward system: current perspectives and future research needs. Nutr Rev. 2015 May;73(5):296-307.
[26]Begg DP, Woods SC. The endocrinology of food intake. Nat Rev Endocrinol. 2013 Oct;9(10):584-97.
[27]Goldstone AP et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009 Oct;30(8):1625-35.
[28]Siep N et al. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav Brain Res. 2009 Mar 2;198(1):149-58.
[29]Haase L, Cerf-Ducastel B, Murphy C. Cortical activation in response to pure taste stimuli during the physiological states of hunger and satiety. Neuroimage. 2009 Feb 1;44(3):1008-21.
[30] Stice E, Burger K, Yokum S. Caloric deprivation increases responsivity of attention and reward brain regions to intake, anticipated intake, and images of palatable foods. Neuroimage. 2013 Feb 15;67:322-30.
[31] de Araujo IE et al. Food reward in the absence of taste receptor signaling. Neuron. 2008 Mar 27;57(6):930-41.
[32]Avena NM, Rada P, Hoebel BG. Sugar and fat bingeing have notable differences in addictive-like behavior. J Nutr. 2009 Mar;139(3):623-8.
[33]Berthoud HR, Zheng H, Shin AC. Food reward in the obese and after weight loss induced by calorie restriction and bariatric surgery. Ann N Y Acad Sci. 2012 Aug;1264(1):36-48.
[34]Pannacciulli N et al. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr. 2006 Oct;84(4):725-31.
[35]DelParigi A et al. Successful dieters have increased neural activity in cortical areas involved in the control of behavior. Int J Obes (Lond). 2007 Mar;31(3):440-8.
[36]Burger KS, Stice E. Relation of dietary restraint scores to activation of reward-related brain regions in response to food intake, anticipated intake, and food pictures. Neuroimage. 2011 Mar 1;55(1):233-9.
[37]Stice E, Davis K, Miller NP, Marti CN. Fasting increases risk for onset of binge eating and bulimic pathology: a 5-year prospective study. J Abnorm Psychol. 2008 Nov;117(4):941-6.
[38]Ogawa R et al. Chronic food restriction and reduced dietary fat: risk factors for bouts of overeating. Physiol Behav. 2005 Nov 15;86(4):578-85.
[39]Gurr MI et al. Adipose tissue cellularity in man: the relationship between fat cell size and number, the mass and distribution of body fat and the history of weight gain and loss. Int J Obes. 1982;6(5):419-36. PMID: 7174187.
[40]Yang MU, Presta E, Björntorp P. Refeeding after fasting in rats: effects of duration of starvation and refeeding on food efficiency in diet-induced obesity. Am J Clin Nutr. 1990 Jun;51(6):970-8.
[41]Arner P. Control of lipolysis and its relevance to development of obesity in man. Diabetes Metab Rev. 1988 Aug;4(5):507-15. PMID: 3061758.
[42]MacLean PS et al. Peripheral metabolic responses to prolonged weight reduction that promote rapid, efficient regain in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol. 2006 Jun;290(6):R1577-88.
[43]Sclafani A, Ackroff K. The relationship between food reward and satiation revisited. Physiol Behav. 2004 Aug;82(1):89-95.
[44]Berthoud HR, Lenard NR, Shin AC. Food reward, hyperphagia, and obesity. Am J Physiol Regul Integr Comp Physiol. 2011 Jun;300(6):R1266-77.
[45]Speakman JR. Thrifty genes for obesity, an attractive but flawed idea, and an alternative perspective: the 'drifty gene' hypothesis. Int J Obes (Lond). 2008 Nov;32(11):1611-7.
08/15/2025
The Spread of Dieting May Be Fueling the Rise in Obesity
Summary
(1) Alongside the rising prevalence of being overweight and being obese, the prevalence of dieting has also steadily increased over the past several decades. There is concern that dieting may paradoxically be contributing to obesity.
(2) Several observational studies suggest that there is at least a partial causal relationship between dieting and weight gain, while some researchers argue that dieting is merely a proxy marker for a tendency to overeat.
(3) Some prospective studies have found that dieting for weight loss—particularly among adolescents, middle-aged women, and individuals within the normal weight range—is one of the strongest predictors of future weight gain. Social pressure to achieve an ideal slim body, along with a fear of fatness, may drive some people to engage in extreme calorie-restricted diets.
(4) In prospective studies of adolescents, the largest ten-year increases in BMI were observed in both males and females who consistently engaged in unhealthy weight control behaviors, such as skipping meals or eating very little.
Some researchers point out that dieting in adolescence is likely to promote behavior patterns—such as binge eating, skipping breakfast, and insufficient vegetable intake—that are counterproductive to long-term weight management.
<Conclusion>
(5) Some observational studies suggest that dieting itself, independent of genetic factors, may predict an increase in BMI. In long-term clinical studies such as starvation experiments, which involve severe caloric restriction, weight overshooting has also been observed after the restrictions were lifted.
(6) (My thoughts) Not all diets, but some forms of dietary restriction and unhealthy weight control behaviors—such as skipping meals—practiced by certain individuals may be contributing to overall weight gain at the population level.
(7) Weight loss through dietary restriction has been found to trigger a biological starvation response—accompanied by metabolic, hormonal, and neurological changes—which may help explain why body weight can end up higher than the pre-diet level.
【 Full Text 】
-
Contents
-
- Recent trends in dieting and obesity
- Problems and points to note in the observational study
- Is there a causal relationship between dieting and weight gain?
- Conclusion: My thoughts
<Introduction>
In recent years, more and more people around the world are going on diets to lose weight. However, some have raised concerns that dieting itself may be contributing to the rise in obesity.
For example, have you ever noticed that actresses or female TV announcers seem to have gained weight compared to how they looked in the past? To me, it’s hard to believe they’re simply overeating. On the contrary, I suspect they may actually be dieting—such as skipping meals or eating smaller portions—because they don’t want to gain weight.
In this article, I’d like to explore whether there is a link between the prevalence of dieting and the rise in obesity, based on findings from observational and clinical studies.
1. Recent trends in dieting and obesity
(1) In 1992, a panel of experts convened by the U.S. National Institutes of Health concluded: “With continued participation in weight-loss programs conducted in controlled settings, participants typically lose about 10% of their body weight. However, within one year after weight loss, one-third to two-thirds of the weight is regained, and within five years, almost all of it is regained [1].”
In addition, studies on long-term outcomes have shown that at least one-third of dieters regain more weight than they lost [2], raising concerns that dieting may paradoxically be promoting exactly the opposite of what it is intended to achieve[2,3].
(2) The notion that dieting to lose weight is counterproductive for weight control in that people may regain more fat than they lose through each cycle of weight loss/regain was embodied in the 1983 book “Dieting Makes You Fat[4].” Since then, whether dieting leads to weight gain remains a controversial and frequently debated topic among scientists [5,6,7].
(3) As of 1998, Americans spent over $33 billion annually on diet-related products and services [8]. Nevertheless, the prevalence of obesity (BMI ≥ 30) has steadily increased—from 30.5% in 2000 to 35.7% in 2010, and 42.4% in 2018 [9].
The prevalence of dieting has also continued to rise over the past several decades (see Table 1) alongside the increasing rates of obesity [10].
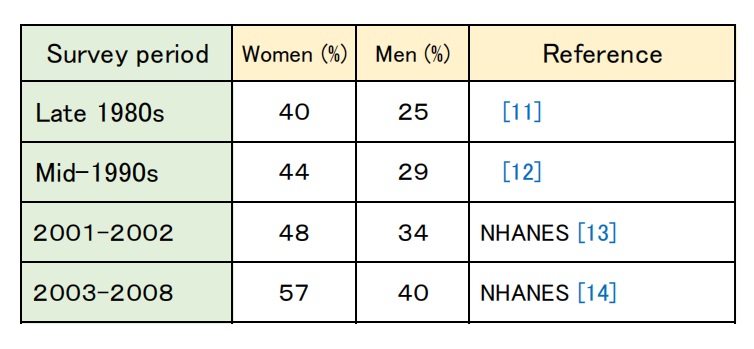
Table 1: Trends in the prevalence of dieting in the U.S.
In the United Kingdom as well, the age-standardized prevalence of weight loss attempts rose from 39% in 1997 to 47% in 2013.
The number of people trying to lose weight has increased each year across all BMI categories [15].
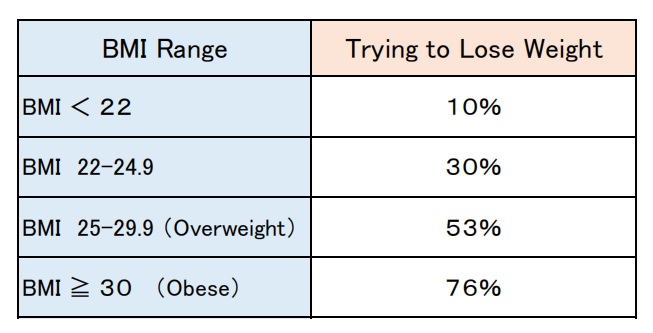
Table 2: Prevalence of weight loss attempts by BMI category (2013,UK)
(4) While some researchers have suggested that the link between dieting and weight gain may be at least partially causal [16,17], others have argued that dieting is a proxy marker for a tendency to overeat and that without dieting, individuals would gain even more weight [18].
(5) Several prospective studies suggest that dieting to lose weight during adolescence [17,19,20], among middle-aged women [21], or by individuals who were initially within the normal weight range [5,21,22] are the strongest and most consistent predictors of future weight gain.
(6) A 10-year prospective study conducted in Minnesota (1998–2009) tracked dieting behaviors and BMI changes among adolescents every five years. A total of 1,902 participants (819 males and 1,083 females) completed the study.
Both males and females who consistently engaged in dieting and unhealthy weight control behaviors (such as skipping meals, eating very little, using food substitutes or diet pills) at both the start of the study (Time 1) and five years later (Time 2) had higher baseline BMIs, and showed greater increases in BMI after ten years (Time 3), as compared to those who did not diet.

Photo Credit: Freepik (photo by Prostooleh)
(*1) 43.7% of females and 18.7% of males reported persistent use (at both Time 1 and Time 2) of unhealthy weight control behaviors.)
In particular, "eating very little" and "skipping meals" were by far the most commonly reported behaviors, and both predicted statistically significant greater BMI increases in both females and males.
Of particular concern was that respondents who were overweight (25 ≤ BMI < 30) at Time 1 and continued to engage in dieting or unhealthy weight control behaviors, showed substantial increases in BMI.
For example, overweight females who practiced unhealthy weight control behaviors at both Time 1 and Time 2 experienced an average BMI increase of 5.19 units over the 10-year study period, whereas those who did not use any unhealthy behaviors saw only a 0.15-unit increase [17].
(7) The 1998 U.S. National Health Interview Survey investigated the prevalence of weight loss strategies among U.S. adults (see Table 3).
Only one-third of those attempting to lose weight reported eating fewer calories and exercising more [23].
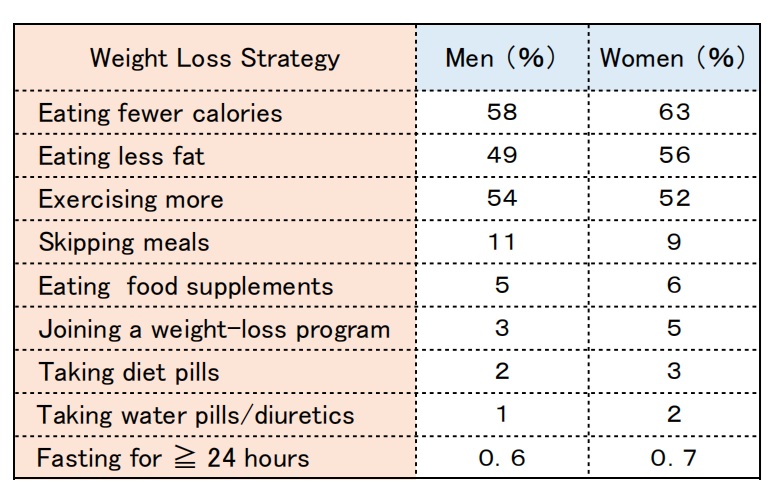
Table 3: The prevalence of weight loss strategies among U.S. adults (1998)
2. Problems and points to note in the observational study
While the majority of longitudinal observational cohort studies have shown subsequent weight gain among self-report dieters, some of the findings have suggested that dieting predicts both weight loss and weight gain, and the results have not always been consistent.
I would like to explore the possible reasons for this and other important points to consider.
(1) What kind of dieting was involved?
Many studies have examined whether participants were dieting or their history of dieting at the start, but not so many have investigated specific dieting behavior.
Of course, people who followed a healthy and sustainable approach to weight loss—e.g., eating more vegetables, reducing ultra-processed foods, eating breakfast, and exercising—may have been able to maintain their weight loss. On the other hand, those who followed the wrong kind of diet that only produced short-term results may have ultimately ended up failing.
(2) Study duration and timing of dieting
In some studies, dieting status was assessed only at the beginning, and changes in BMI were then tracked several years later (e.g., after 2, 5, or 10 years). However, from a homeostasis perspective, individuals who were dieting at the start of the study may already have had a body weight below their original weight (meaning set-point weight) .
Additionally, if someone begins (or stops) dieting during the study period, or starts dieting shortly before the study ends, it may be difficult to accurately determine the causal relationship between dieting and weight change.
(3) BMI tends to increase during adolescence
An increase in BMI does not necessarily mean an increase in body fat. During adolescence (from junior high through high school), muscle mass also increases significantly, so a certain amount of BMI gain is not unusual. This is something to keep in mind when conducting observational studies in this age group.
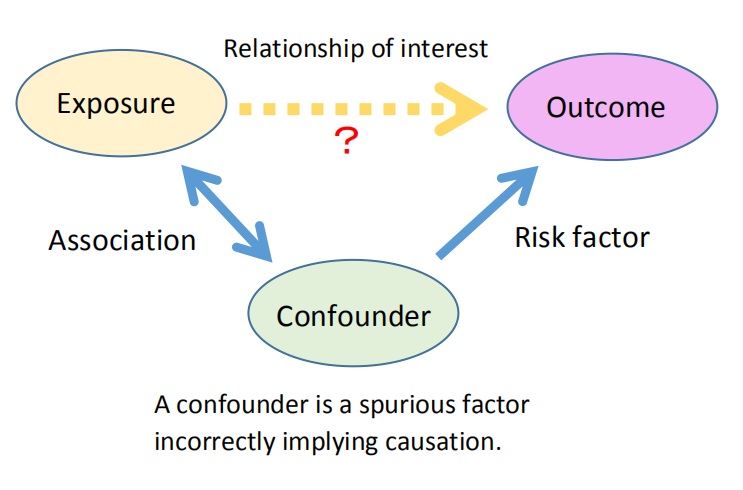
(4) Confounding factors
Confounding factors are variables that can influence the relationship between a specific exposure and an outcome.
For example, when studying the link between alcohol consumption and cancer, smoking is known to increase cancer risk as well. Since people who drink alcohol often smoke, smoking becomes a confounding factor.
When examining the causal relationship between dieting and obesity, it’s important to account for other potential confounding factors (*2).
(*2) For example: alcohol consumption, smoking cessation, lack of physical activity, insufficient intake of calcium or micronutrients, socioeconomic status, childbirth, and sleep deprivation are all associated with weight gain.
3. Is there a causal relationship between dieting and weight gain?
Throughout this blog, I’ve explained that calorie-restricted diets—such as skipping meals—may induce intestinal starvation, which could lead to weight gain-meaning an increase in the body’s set-point weight. So it’s not at all surprising that the rise in dieting may be linked to the rise in obesity.
However, this time, I’d like to set aside my own ideas and explore this causal relationship based on what can be interpreted from observational and clinical studies.
(1) First, let’s look at the claim that “dieting is merely a proxy marker for a tendency to overeat, and without dieting, people would gain even more weight.”
In the 10-year study of adolescents discussed in Section 1-(6), researchers were able to track the trajectories of those who were dieting at the start (Time 1) but either stopped dieting or continued dieting five years later (Time 2).
They found that those who stopped dieting gained significantly less weight compared to those who continued dieting. This finding does not support the claim that people would gain more weight if they didn’t diet[17].
(2) Another idea regarding dieting and obesity is that ”it’s not dieting itself that causes subsequent weight gain, but rather that people who are genetically prone to obesity are more likely to go on a diet[6].” In other words, even if the dieting is unsuccessful, the weight gain is attributed to genetic factors.
This claim was also contradicted by the results of a 10-year study on adolescents.
Among girls who were overweight at baseline (Time 1) and continued dieting or engaged in unhealthy weight control behaviors (such as skipping meals or eating very little) at both Time 1 and Time 2, BMI increased by 5.19 units over the 10-year period. In contrast, overweight girls who did not engage in any dieting or unhealthy weight control behaviors showed only a 0.15-unit increase in BMI. This suggests that dieting itself may have had a significant impact on weight gain[17].
In addition, a longitudinal twin study conducted in Finland examined weight changes in identical and fraternal twins who differed in the number of lifetime intentional weight loss episodes of more than five kg. The findings suggests that frequent intentional weight loss (dieting) may lead to weight gain over time, independent of genetic factors[16].
(3) Why is dieting a stronger predictor of weight gain among adolescents and individuals with a normal weight?
According to previous studies, many young people are concerned about their body shape and size due to social pressure to achieve an ideal lean figure[28]. Additionally, for individuals who are within the normal weight range (BMI under 25) but have recently experienced gradual weight gain, dieting is motivated by a fear of fatness rather than by a desire to become thin[29].
Several studies have pointed out the prevalence of unhealthy weight control behaviors among adolescents (especially girls), such as skipping meals, eating very little, using appetite suppressants or laxatives, vomiting, binge eating, and incidental exercise[17, 30].
A five-year longitudinal study of adolescents found that dieting was associated with an increase in binge eating (males and females), a decrease in breakfast consumption (males and females) , reduced intake of fruits and vegetables (females), and decreased physical activity (males). In other words, dieting among adolescents may actually increase the risk of unhealthy eating and activity behaviors, potentially leading to counterproductive patterns for long-term weight management[19].
(4) Clinical studies, starvation experiments
Weight overshooting in normal-weight subjects during experimental semi-starvation and the recovery period, has been documented in the classic Minnesota Starvation Experiment (1945–46) and the U.S. Army Ranger multi-stress experiments[31].
In the Minnesota study, healthy men with an average weight of 69.3 kg lost more than 25% of their body weight during six months of semi-starvation. However, during the ad libitum refeeding period following 12 weeks of restricted rehabilitation, a hyperphagic response (incessant sensation of desire to eat)persisted, and as a result, their weight eventually exceeded their pre-starvation level[32,33].
In more recent years, similar body weight and fat overshooting have also been reported in young men at the US Army Ranger School. They lost about 12% of their body weight during 8–9 weeks of training under multiple stressors, including energy deficit and sleep deprivation, but at week 5 in the post-training recovery phase, their body weight had overshot by 5 kg, primarily due to increased fat mass[34].
<Summary of this section>
● Some observational studies suggest that dieting itself, regardless of genetic factors, may predict an increase in BMI.
● Of particular concern for weight gain is unhealthy dieting in adolescents and normal weight individuals.
● Some researchers point out that unhealthy dieting is likely to encourage behavioral patterns that are counterproductive to long-term weight management.
● In long-term clinical studies involving severe caloric restrictions, weight overshooting has been observed after the restrictions were lifted.
4. Conclusion: My thoughts
Not all diets lead to weight gain.
Some people succeed in losing weight and keeping it off by adopting a healthy eating pattern that suits them—for example, starting the day with breakfast, eating a balanced diet, reducing refined carbohydrates and ultra-processed foods, increasing vegetable intake, and exercising.
For instance, a systematic review analyzing six prospective cohort studies on the Mediterranean diet found that adherence to the Mediterranean diet was inversely associated with the risk of overweight and obesity, as well as with weight gain over five years[35].

Photo Credit: Freepik (Photo by Katemangostar)
However, obesity prevention and treatment are still often discussed solely from the perspective of “eating fewer calories and burning more,” leading many people to choose low-fat diets, skip breakfast or lunch, or endure long hours of hunger with nothing but small portions of fast food.
In particular, some young people, driven by social pressure or a fear of fatness, resort to extreme calorie restriction—such as skipping meals or eating very little—which raises concerns about both their health and weight gain over time[17].
While it’s difficult to prove causality through observational studies, I believe that the behaviors (such as dietary restriction) practiced by some individuals may be contributing to overall weight gain at the population level.
From an evolutionary perspective, it makes sense that the body would conserve its energy stores by reducing energy expenditure during times of scarcity, such as famine, and then quickly replenish those stores (body fat) when food is abundant [36]. In modern societies where tasty, easily digestible foods—such as refined carbohydrates and ultra-processed foods—are widely available, I believe that extreme dietary restriction can sometimes backfire.
According to previous studies, weight loss through dietary restriction has been found to trigger a biological starvation response—accompanied by metabolic, hormonal, and neurological changes—[37]which may help explain why body weight can end up increasing beyond the pre-diet level.
In the next article, I’d like to discuss various mechanisms that may promote weight gain after weight loss, including my intestinal starvation theory.
<References>
[1] Methods for voluntary weight loss and control. NIH Technology Assessment Conference Panel. Ann Intern Med. 1992 Jun 1;116(11):942-9.
[2] Mann T et al. Medicare's search for effective obesity treatments: diets are not the answer. Am Psychol. 2007 Apr;62(3):220-33.
[3] Bacon L, Aphramor L. Weight science: evaluating the evidence for a paradigm shift. Nutr J. 2011 Jan 24;10:9.
[4]Cannon G, Einzig H. Dieting makes you fat. London: Century Publishing; 1983.
[5] Jacquet P et al. How dieting might make some fatter: modeling weight cycling toward obesity from a perspective of body composition autoregulation. Int J Obes (Lond). 2020 Jun;44(6):1243-1253.
[6] Hill AJ. Does dieting make you fat. Br J Nutr. 2004 Aug;92 Suppl 1:S15-8.
[7] Lowe MR. Dieting: proxy or cause of future weight gain? Obes Rev. 2015 Feb;16 Suppl 1:19-24.
[8] Cleland R et al. Commercial weight loss products and programs: what consumers stand to gain and lose. Crit Rev Food Sci Nutr. 2001 Jan;41(1):45-70.
[9] National Center for Health Statistics, National Health and Nutrition Examination Survey, 1999–2018.
[10] Montani JP et al. Dieting and weight cycling as risk factors for cardiometabolic diseases: who is really at risk? Obes Rev. 2015 Feb;16 Suppl 1:7-18.
[11] Williamson DF et al. Weight loss attempts in adults: goals, duration, and rate of weight loss. Am J Public Health. 1992 Sep;82(9):1251-7.
[12]Serdula MK et al. Prevalence of attempting weight loss and strategies for controlling weight. JAMA. 1999 Oct 13;282(14):1353-8.
[13] Weiss EC et al. Weight-control practices among U.S. adults, 2001-2002. Am J Prev Med. 2006 Jul;31(1):18-24.
[14] Yaemsiri S et al. Perceived weight status, overweight diagnosis, and weight control among US adults: the NHANES 2003-2008 Study. Int J Obes (Lond). 2011 Aug;35(8):1063-70.
[15] Piernas C et al. Recent trends in weight loss attempts: repeated cross-sectional analyses from the health survey for England. Int J Obes (Lond). 2016 Nov;40(11):1754-1759.
[16]Pietiläinen KH et al. Does dieting make you fat? A twin study. Int J Obes (Lond). 2012 Mar;36(3):456-64.
[17] Neumark-Sztainer D et al. Dieting and unhealthy weight control behaviors during adolescence: associations with 10-year changes in body mass index. J Adolesc Health. 2012 Jan;50(1):80-6.
[18] Stice E, Presnell K. Dieting and the eating disorders. In: Agras WS, editor. The Oxford Handbook of Eating Disorders. Oxford University Press; USA: 2010. pp. 148–179.
[19] Neumark-Sztainer D et al. Why does dieting predict weight gain in adolescents? : a 5-year longitudinal study. J Am Diet Assoc. 2007 Mar;107(3):448-55.
[20]Viner RM, Cole TJ. Who changes body mass between adolescence and adulthood? Factors predicting change in BMI:1970 British Birth Cohort. Int J Obes (Lond). 2006 Sep;30(9):1368-74.
[21]Korkeila M et al. Weight-loss attempts and risk of major weight gain: a prospective study in Finnish adults. Am J Clin Nutr. 1999 Dec;70(6):965-75.
[22] Sares-Jäske L et al. Self-report dieting and long-term changes in body mass index and waist circumference. Obes Sci Pract. 2019 Mar 26;5(4):291-303.
[23] Kruger J et al. Attempting to lose weight: specific practices among U.S. adults. Am J Prev Med. 2004 Jun;26(5):402-6.
[24] Bild DE et al. Correlates and predictors of weight loss in young adults: the CARDIA study. Int J Obes Relat Metab Disord. 1996 Jan;20(1):47-55. PMID: 8788322.
[25] Coakley EH et al. Predictors of weight change in men: results from the Health Professionals Follow-up Study. Int J Obes Relat Metab Disord. 1998 Feb;22(2):89-96.
[26] French SA et al. Predictors of weight change over two years among a population of working adults: the Healthy Worker Project. Int J Obes Relat Metab Disord. 1994 Mar;18(3):145-54. PMID: 8186811.
[27] Chaput JP et al. Risk factors for adult overweight and obesity in the Quebec Family Study. Obesity (Silver Spring). 2009 Oct;17(10):1964-70.
[28]Field AE et al. Family, peer, and media predictors of becoming eating disordered. Arch Pediatr Adolesc Med. 2008 Jun;162(6):574-9.
[29] Chernyak Y, Lowe MR. Motivations for dieting: Drive for Thinness is different from Drive for Objective Thinness. J Abnorm Psychol. 2010 May;119(2):276-81.
[30] Stice E et al. Naturalistic weight-reduction efforts prospectively predict growth in relative weight and onset of obesity among female adolescents. J Consult Clin Psychol. 1999 Dec;67(6):967-74.
[31] Dulloo AG et al. How dieting makes some fatter: from a perspective of human body composition autoregulation. Proc Nutr Soc. 2012 Aug;71(3):379-89.
[32] Keys A et al. (1950) The Biology of Human Starvation. Minnesota: University of Minnesota Press.
[33] Jason Fung. The Obesity Code. Greystone Books, 2016, Pages 36-39.
[34]Nindl BC et al. Physical performance and metabolic recovery among lean, healthy men following a prolonged energy deficit. Int J Sports Med. 1997 Jul;18(5):317-24.
[35]Lotfi K et al. Adherence to the Mediterranean Diet, Five-Year Weight Change, and Risk of Overweight and Obesity: A Systematic Review. Adv Nutr. 2022 Feb 1;13(1):152-166.
[36] van Baak M. Adaptive thermogenesis during over- and underfeeding in man. Br J Nutr. 2004 Mar;91(3):329-30.
[37] Mann T et al. Promoting Public Health in the Context of the "Obesity Epidemic": False Starts and Promising New Directions. Perspect Psychol Sci. 2015 Nov;10(6):706-10.
03/03/2025
The Rise in Obesity is Closely Linked to the Consumption of Ultra-Processed Foods
Summary
(1) The NOVA classification system, developed in 2009 by a research group at the University of São Paulo, categorizes foods into four groups based on the degree and purpose of processing rather than type or their nutritional content.
【1】Unprocessed or minimally processed foods
【2】Processed culinary ingredients
【3】Processed foods (PFs)
【4】Ultra-processed foods (UPFs)
Ultra-processed foods (UPFs) are formulations made from multiple ingredients and undergo numerous industrial processes. They include sweet or savory snacks, confectioneries, instant foods, processed meat products such as sausages and ham, and most fast foods.
Increased UPF consumption is believed to be linked to rising rates of obesity and diet-related diseases.
(2) UPFs are high in refined carbohydrates, added sugars, salt, saturated fats, and trans fats, making them energy-dense. On the other hand, they are poor sources of fiber, protein, and micronutrients.
Additionally, they often contain flavorings, colorings, emulsifiers, preservatives, and other cosmetic additives.
(3) Since the 1980’s, UPF consumption has surged not only in developed countries but also in developing nations. UPFs now account for more than 50% of total daily energy intake on average in the U.S., U.K., and Canada.
(4) Several studies examining the relationship between UPF consumption and obesity have clearly shown that higher UPF intake is associated with an increased risk of obesity. In contrast, greater consumption of natural foods, such as vegetables, has been found to be inversely correlated with obesity.
(5) A U.S. research group found that individuals with high UPF consumption tend to eat fewer natural foods, such as fruits, vegetables, and fish, leading to a significant decline in overall diet quality.
(6) Processed food diets may result in lower diet-induced thermogenesis (DIT) compared to whole-food diets, potentially increasing net energy intake. Additionally, UPF diets may lead to overeating because they provide less satiety and make individuals feel hungrier than unprocessed food diets.
<My thought>
(7) Generally, UPFs are considered to promote obesity when consumed in excess due to their high energy density. However, I want to highlight the risk of “ultra-processing” itself. Since UPFs are low in nutrients and fiber, and are easily digested, if a diet is skewed toward refined carbohydrates and UPFs while lacking natural foods like vegetables, it can lead to intestinal starvation, potentially raising the body's set-point weight.
(8) It is not an overstatement to say that the sharp rise in being overweight, obesity, and many lifestyle-related diseases coincided with the rapid industrialization of food processing in the 1970’s and 80’s.
(9) The World Obesity Federation (WOF) warns that without policy changes and effective obesity prevention measures, more than half of the global population will be classified as obese or overweight by 2035. In my opinion, instead of focusing solely on “calories,” policies need to emphasize factors such as the degree of food processing, the number of chews, and the digestibility of food.
【 Full text 】
-
Contents
-
- Food classification by NOVA
- The Issues with Ultra-Processed Foods
- Consumption of UPFs and its association with obesity
- Impact of UPFs on overall diet
(1) Decrease in overall diet quality
(2) Increase in net energy intake
(3)Effects on ad libitum energy intake - Why do UPFs cause weight gain?
- Desired future measures
The endless diet wars among factions in various diets—such as low-carb, ketogenic, paleo, low-fat, and vegan—have caused substantial public confusion and fostered mistrust in nutritional science. However, it is not widely known that diverse diets recommendations often share a common piece of advice: to avoid ultra-processed foods[1].
Empirical evidence has shown that the rising obesity rates closely parallel the increased consumption of ultra-processed foods in many countries.
In this discussion, I’d like to explore the reasons behind this and, finally, mention how it relates to my intestinal starvation theory.
1. Food classification by NOVA
NOVA (not a acronym) is the food classification that categorizes foods based on the degree and purpose of food processing, rather than their nutritional content. It was developed in 2009 by a research group at the University of São Paulo in Brazil[2].
Conventional food classification systems categorize foods and ingredients based on their botanical origin or animal species, and nutrient composition.
As a result, whole grains are often grouped with breakfast cereals or cookies, and fresh chicken or pork are often classified alongside chicken nuggets or sausages.
When considering their their impact on health and disease, conventional food classifications no longer worked well[3].

NOVA classifies foods into four groups based on the nature, extent, and purpose of the industrial processing they undergo.
(1)Unprocessed or minimally processed foods
Fresh fruits and vegetables, and other natural foods (such as grains, milk, fish, and meat) that have undergone processes like removing inedible parts, drying, grinding, pasteurization, chilling, freezing, or vacuum-packaging.
(2)Processed culinary ingredients
Substances derived from Group 1 foods or from nature through processes that include pressing, refining, milling, or drying, such as oils, butter, sugar, and salt. These processed culinary ingredients are typically not consumed on their own.
(3)Processed foods (PFs)
These are typically made by adding Group 2 substances to Group 1 foods. Examples include canned vegetables, fruit in syrup, canned fish, cheese, and freshly made breads.
(4)Ultra-processed foods (UPFs)
These are formulations created by combining many ingredients and undergoing a sequence of industrial processes. Examples include breakfast cereals, soft drinks and fruit juices, sweet or savory snacks, confectioneries, instant foods, reconstituted meat products such as sausages and nuggets, and most fast food items[3,4].
2. The issues with ultra-processed foods
Food processing, in essence, refers to “various operations by which raw foodstuffs are made suitable for consumption, cooking, or storage,” and virtually all foods undergo some form of processing before being eaten. Therefore, processing itself is not inherently bad.
However, ultra-processed foods are not modified foods but formulations made mostly or entirely from substances derived from foods and additives through multiple industrial processes, with little to no Group 1 natural foods[3].
These foods are high in refined grains, added sugars, salt, saturated fats, and trans fats, making them energy-dense. On the other hand, they are poor sources of dietary fiber, protein, and micronutrients.
Additionally, flavorings, colorings, emulsifiers, non-sugar sweeteners, and other cosmetic additives are often added to these products to mask undesirable qualities of the final product[5].
Nevertheless, since the 1980s, the consumption of ultra-processed foods (UPFs) has rapidly increased not only in developed countries but also in developing nations, largely driven by transnational corporations.

UPFs are highly appealing because they are hyper-palatable and addictive, inexpensive, have a long shelf life, and can be consumed anytime, anywhere[3].
The evidence based on the NOVA classification so far suggests that the decline in minimally processed foods and home cooking, alongside the replacement of food supplies with ultra-processed foods, is associated with unhealthy nutritional profiles and an increase in several diet-related diseases[3].
3. Consumption of UPFs and its association with obesity
In studies on adults reporting the proportion of total daily energy intake from UPFs, the highest levels (on average) were reported in the USA (55.1–56.1%), followed by the UK (53–54.3%), Canada (45.1–51.9%), France (29.9–35.9%), Brazil (20–29.6%), Spain (24.4%), and Malaysia (23%)[6].
A cross-sectional study (2005–2014) on American adults, who were found to consume an average of 56.1% of their total energy intake from UPFs, revealed significant differences across quintiles. Those in the highest quintile (Note 1) consumed 84.5% of their total energy intake from UPFs, while those in the lowest quintile accounted for 25.4%[7].
(Note 1: Quintile refers to one of five equal measurements that a set of things can be divided into)
♦A cross-sectional time series study conducted in fifteen Latin American countries revealed that sales of UPFs were associated with changes in body weight in twelve of these countries from 2000 to 2009[8].
A cross-sectional study based on data from Brazil’s 2008–2009 Household Budget Survey found that household availability of UPFs was positively correlated with both the average BMI and obesity prevalence. Those in the highest quartile (Note 2) of household consumption of UPFs were 37% more likely to be obese compared to those in the lowest quartile[9].
(Note 2: Quartile refers to one of four equal measurements that a set of things can be divided into)
♦A study involving 6,143 participants from the UK National Diet and Nutrition Survey (2008–2016) classified foods recorded in four-day food diary according to the NOVA system. Consumption of UPFs was associated with increases in BMI, waist circumference, and obesity rates in both men and women. For every 10% increase in UPF consumption, the obesity rate increased by 18%.
Higher consumption of UPFs was observed among men, white British individuals, smokers, younger people, and those in lower social class groups[10].
♦A cross-sectional study involving 19,363 adults aged 18 and older from the 2004 Canadian Community Health Survey, found that individuals in the highest quintile of UPF consumption were 32% more likely to be obese compared to those in the lowest quintile. Higher UPF intake was associated with being male, younger age, lower educational attainment, physical inactivity, smoking, and being born in Canada[4].
♦A prospective cohort study conducted since 1999 among graduates of the University of Navarra in Spain tracked 8,451 participants who were not overweight at baseline for about nine years. Those in the highest quartile of UPF consumption had a 26% higher risk of developing overweight or obesity compared to those in the lowest quartile.
Moreover, on average, they consumed more fast food, fried foods, processed meats, and sugar-sweetened beverages, while, in contrast, their vegetable intake was the lowest. A higher consumption of UPFs was associated with lower adherence to the Mediterranean diet[11].
3. Impact of UPFs on overall diet
(1) Decrease in overall diet quality
♦A U.S. research group analyzed data on 5,919 children and 10,064 adults from the National Health and Nutrition Examination Survey (2015-2018) to investigate the relationship between UPF consumption and overall diet quality. Diet quality was assessed using the American Heart Association (AHA) diet score and Healthy Eating Index (HEI)-2015 score.
The estimated proportion of children with a poor diet gradually increased from 31.3 % in the lowest quintile of UPF consumption to 71.6 % in the highest quintile. Similarly, among adults, this proportion rose from 18.1 % in the lowest quintile to 59.7 % in the highest quintile.
As UPF intake increased, the consumption of healthy foods such as fruits, vegetables, nuts, and fish significantly decreased, while the intake of unhealthy foods such as refined grains, sugar-sweetened beverages, and added sugars increased.

The research group concluded that higher consumption of UPFs was associated with substantially lower diet quality among children and adults. These findings were consistent with previous studies conducted in several countries[12].
♦An Italian research group hypothesized that meal timing could also be linked to food processing and conducted a study to test this. They analyzed data on 8,688 individuals from the Italian Nutrition & Health Survey (INHES), conducted between 2010 and 2013. Subjects were classified as early or late eaters based on the population’s median timing for breakfast, lunch, and dinner.
Results showed that late eaters (breakfast after 7 AM, lunch after 1 PM, and dinner after 8 PM) were less likely to consume unprocessed or minimally processed foods compared to early eaters, while they consumed more processed foods (PFs) and UPFs. Late eating was also inversely associated with adherence to the Mediterranean diet[13].
The Mediterranean diet, which primarily consists of fruits, vegetables, legumes, nuts, olive oil, and fish, has been shown to be linked to reduced weight gain[14].
(2) Increase in net energy intake
A group of researchers in the U.S. conducted a crossover comparative study to ascertain the net energy intake of two different diets: one consisting of specific processed foods and the other of an isocaloric whole-food (WF) diet. Eighteen subjects consumed two types of sandwiches with the same calorie content but differing in processing levels.
The WF meal consisted of multigrain bread (containing whole sunflower seeds and whole-grain kernels) and cheddar cheese, while the PF meal consisted of white bread and a processed cheese product.
In this study, diet-induced thermogenesis (DIT)(Note 3) after consuming the PF meal was 46.8 % lower than that of the WF meal. The researchers concluded that this difference in DIT led to a 9.7 % increase in net energy-gain for the PF meal[15].
(Note 3) Diet induced thermogenesis (DIT) is the process by which the body increases its energy expenditure for several hours in response to food intake.
Regarding the significant reduction in diet-induced thermogenesis (DIT) observed with the PF meal, the researchers provided the following analysis:
Compared to whole foods, PFs are characterized by lower nutrient density (a lower content and diversity of nutrients per calorie), less dietary fiber, and an excess of simple carbohydrates. As a result, PFs are structurally and chemically simpler than whole foods, making them easier to digest[15,16].
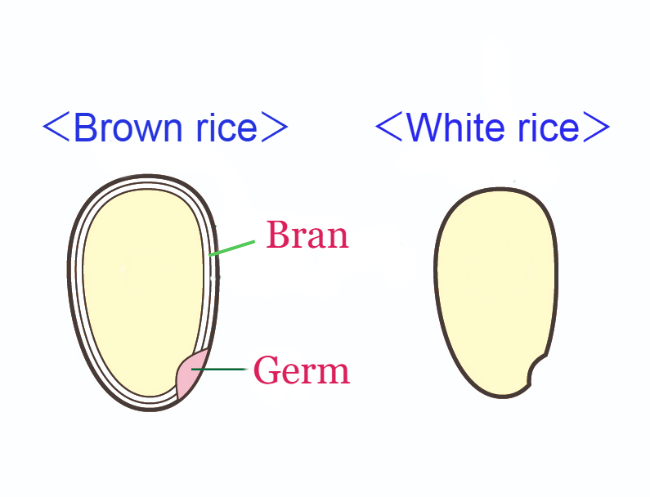
In grain refinement, most of the bran and germ are removed, resulting in the loss of nutrients (such as vitamins, minerals, and proteins), fiber, and phenols they provide.
Consequently, PFs tend to have fewer metabolites, leading to reduced enzyme production and peristalsis (Note 4), easier absorption, and less secondary metabolism—all of which contribute to lower DIT[15, 17].
Additionally, the loss of fiber reduces meal bulk and tends to slow satiety, both of which can lead to an increase in daily caloric intake[15, 18].
(Note 4:the repeated movements made by the muscle walls in the digestive tract tightening and then relaxing)
(3)Effects on ad libitum energy intake
A group of U.S. researchers conducted a randomized controlled trial to determine the effects of ultra-processed versus unprocessed diets on ad libitum energy intake in twenty adults with stable body weight. Subjects were randomly assigned to either the ultra-processed or calorie-matched unprocessed diet for two weeks, followed by the alternate diet for another two weeks.
In this experiment, participants were allowed to eat additional food after meals. As a result, they consumed more energy (459±105 kcal /day) during the ultra-processed diet and gained 0.4±0.1 kg of body fat. In contrast, they lost 0.3±0.1 kg of body fat during the unprocessed diet.
Fasting blood tests showed that levels of the appetite-suppressing hormone peptide YY (PYY) increased during the unprocessed diet as compared with both the ultra-processed diet and baseline. Also, levels of the hunger hormone ghrelin decreased during the unprocessed diet compared to baseline. This suggests that participants felt less hungry during the unprocessed diet, whereas the ultra-processed diet provided less satiety, making participants feel hungrier[19].
4. Why do UPFs cause weight gain?
I would like to explain the link between consumption of UPFs and an increase in obesity from the perspective of intestinal starvation.
Since the development of the NOVA classification, various studies have focused on the degree of food processing rather than calorie content, which is noteworthy.
In the past, factors such as refined carbohydrates, fast food, late-night eating, lack of vegetables, wealth, and poverty have been discussed as causes of being overweight. However, how these factors interact had not been clearly proven.
This time, research has shown that increased UPF consumption itself is linked to an overall decline in diet quality, lack of vegetables, late-night eating, and lower-income groups in developed countries.
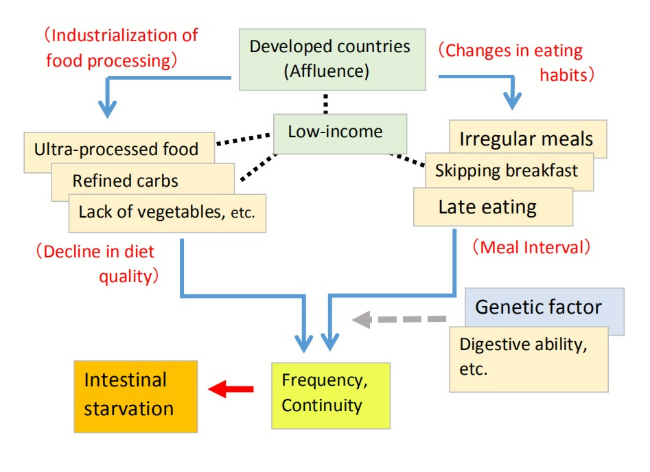
Through this blog, I have explained that a combination of factors—such as an unbalanced diet leaning toward refined carbohydrates and UPFs, a lack of vegetables, and an irregular lifestyle—can induce intestinal starvation, potentially raising the body's set-point weight.
UPFs are often high in energy density, so they are generally believed to promote obesity when overeaten. However, I believe the biggest issue is the "ultra-processing" itself, as these foods are digested and absorbed more quickly than natural foods.
If the diet is skewed toward refined carbohydrates and UPFs, and the overall diet quality declines, blood sugar levels can fluctuate sharply. Additionally, since these foods leave nothing behind in the intestines after digestion, intestinal starvation can be triggered (Note 5).
The fact that adherence to the Mediterranean diet and the consumption of natural foods like vegetables are inversely correlated with obesity supports my perspective.
In Japan, In my opinion, the consumption of UPFs such as instant foods, cookies, sweet bread, and chocolate confection is not necessarily low. However, one reason Japan’s obesity rate is lower than in Western countries may be its rice-based food culture, and the fact that many Japanese people still follow traditional eating habits.
(Note 5: Foods high in fat, such as ice cream, take longer to digest and may help prevent intestinal starvation.)
5. Desired future measures
The World Obesity Federation (WOF) warns that if policy directions remain unchanged and no effective measures are taken to prevent and treat obesity, more than half of the global population will be classified as overweight or obese by 2035.
I think this suggests that past policies focused solely on "calories in/ calories out" have not been particularly effective.
What we need now is a shift in focus from "calories" to factors such as the degree of food processing, the number of chews, and digestibility in policymaking.
With traditional dietary habits in many parts of the world, minimally processed natural foods were gradually digested in the digestive tract, allowing energy and nutrients to enter the bloodstream over several hours, while indigestible components like fiber helped maintain gut health.
Even if refined carbohydrates—such as white rice or bread—were included, the overall dietary quality likely remained high.
Even when people felt hungry, fiber and other undigested matter remained in the gut. As a result, there was little need to worry about caloric intake.

However, many people today prefer soft foods that require little chewing and have become overly reliant on refined carbohydrates and UPFs. These diets are low in nutrients and fiber, making them easy to digest, allowing the body to absorb large amounts of energy with minimal effort.
Moreover, once all the food is fully digested in the intestines, a signal that “there is no food” might be sent to the brain.
Such dietary habits were likely rare, if not nonexistent, in human history—at least until around 1970. It is not an overstatement to say that the sharp rise in being overweight, obesity, and many lifestyle-related diseases coincided with the rapid industrialization of food processing in the 1970’s and 80’s.
<References>
[1]Katz DL, Meller S. Can we say what diet is best for health? Annu Rev Public Health. 2014;35:83-103.
[2]Monteiro CA et al. NOVA. The star shines bright. Food classification. Public Health. World Nutr. J. 2016, 7, 28–38.
[3]Monteiro CA et al. The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018 Jan;21(1):5-17.
[4]Nardocci M et al. Consumption of ultra-processed foods and obesity in Canada. Can J Public Health. 2019 Feb;110(1):4-14.
[5]Fiolet T et al. Consumption of ultra-processed foods and cancer risk: results from NutriNet-Santé prospective cohort. BMJ. 2018 Feb 14;360:k322.
[6]Elizabeth L et al. Ultra-Processed Foods and Health Outcomes: A Narrative Review. Nutrients. 2020 Jun 30;12(7):1955.
[7]Juul F et al. Ultra-processed food consumption and excess weight among US adults. Br J Nutr. 2018 Jul;120(1):90-100.
[8]Ultra-processed food and drink products in Latin America: trends, impact on obesity, policy implications. Pan American Health Organization, Washington (DC) (2013)
[9]Canella DS et al. Ultra-processed food products and obesity in Brazilian households (2008-2009). PLoS One. 2014 Mar 25;9(3):e92752.
[10]Rauber F et al. Ultra-processed food consumption and indicators of obesity in the United Kingdom population (2008-2016). PLoS One. 2020 May 1;15(5):e0232676.
[11]Mendonça RD et al. Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr. 2016 Nov;104(5):1433-1440.
[12]Liu J et al. Consumption of Ultraprocessed Foods and Diet Quality Among U.S. Children and Adults. Am J Prev Med. 2022 Feb;62(2):252-264.
[13]Bonaccio M et al. Association between Late-Eating Pattern and Higher Consumption of Ultra-Processed Food among Italian Adults: Findings from the INHES Study. Nutrients. 2023 Mar 20;15(6):1497.
[14]Beunza JJ et al. Adherence to the Mediterranean diet, long-term weight change, and incident overweight or obesity: the Seguimiento Universidad de Navarra (SUN) cohort. Am J Clin Nutr. 2010 Dec;92(6):1484-93.
[15]Barr SB, Wright JC. Postprandial energy expenditure in whole-food and processed-food meals: implications for daily energy expenditure. Food Nutr Res. 2010 Jul 2;54.
[16]Fereidoon Shahidi. Nutraceuticals and functional foods: Whole versus processed foods. Trends in Food Science & Technology, Volume 20, Issue 9, 2009, Pages 376-387.
[17]Secor SM. Specific dynamic action: a review of the postprandial metabolic response. J Comp Physiol B. 2009 Jan;179(1):1-56.
[18]Roberts SB. High-glycemic index foods, hunger, and obesity: is there a connection? Nutr Rev. 2000 Jun;58(6):163-9.
[19]Hall KD et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019 Jul 2;30(1):67-77.e3.
10/14/2024
The Increasingly Important "Set-Point" Theory of Body Weight: What are the Environmental and Behavioral Factors?
Summary
■In 1953, Kennedy proposed that body fat storage is regulated. In 1982, nutritionists William Bennett and Joel Gurin expanded on Kennedy’s idea and developed the set-point theory. This may help explain the recurring failure of long-term dieting.
■When an individual loses weight, the body significantly reduces energy expenditure beyond what would be expected based on changes in body composition and the thermic effect of food. It also increases appetite through hormonal regulation, and alters food preferences to drive the body weight back toward a predetermined set-point range.
■Since obese individuals also exhibit these compensatory metabolic adjustments in response to dietary restriction, obesity may be considered a natural physiological state for some people.
■This body’s set-point is thought to be established early in life and remains relatively stable unless altered by specific life changes, such as marriage, childbirth, menopause, aging, or illness.
■As demonstrated in overfeeding experiments conducted in Vermont in the 1960’s, even weight gain from temporary overeating can trigger compensatory mechanisms that bring body weight back toward its set-point range.
■However, this set-point model of body weight does not explain the global rise in obesity since around 1970. Some researchers point out that while metabolic resistance to sustaining a reduced body weight is strong, its resistance to sustained fat gain may weaken over time with prolonged consumption of high-calorie foods.
< My thoughts >
■As some researchers have suggested, whether obesity as a chronic disease can be cured is depend on understanding how genetic and environmental factors interact to adjust the body's set-point weight.
■Since it is generally believed that a positive energy balance is necessary for weight gain, some researchers suggest that today’s environment of continuous high-calorie food intake may result in irreversible weight gain, which also suggests an increase in set-point weight, but I have a different view.
■There are two types of weight gain. Weight gain from temporary overeating is due to a positive energy balance, but this is usually short-term, and body weight is likely to return to its set-point.
On the other hand, I believe that long-term, irreversible weight gain, which suggests an increase in set-point weight, is more likely triggered by a negative energy balance or internal signals of “food scarcity,” similar to cases where weight increases even more after periods of starvation or dieting.
■Since 1970, one notable change in our living environment is what I call “intestinal starvation.” In many parts of the world before 1970, even if people went a whole day without eating until the next meal, there were likely still fibers and other undigested substances left in the intestines.
However, in today’s society, where easily digestible foods—such as refined carbohydrates, certain kinds of protein, and [ultra-]processed foods—are widely consumed, intestinal starvation can be induced in as little as 8 to 10 hours depending on how one eats. Through the gut-brain axis, this may send a signal to the brain indicating "there is no food."
※For an explanation of why intestinal starvation may lead to irreversible weight gain—suggesting an increase in body's set-point weight—please see Section 4.
【 Full text 】
-
Contents
-
- Advances in understanding “set-point” theory
- Problems with the set-point model
- Environmental and behavioral factors influencing weight set-point
- Why intestinal starvation increases one’s set-point weight
<How to prove my theory>
- Advances in understanding “set-point” theory
As I've explained in previous blog posts, I believe that each person has an individual “set-point” for their weight, and that understanding how this set-point increases is the key to addressing the issue of obesity.
This time, I would like to share my thoughts on recent advances in research regarding the set-point theory of body weight, as well as the environmental and behavioral factors that influence it.
1. Advances in understanding “set-point” theory
<Obesity and weight loss attempts>
♦A fat person who insists that a lean friend has consistently eaten more than the fat person does, may well be telling the truth.(*snip*)
The group of obese patients who are greatly in need of our understanding are those who keep to a calorie intake of perhaps 1000 kcal per day, yet lose less than one kg per week. There is no doubt whatsoever that such people exist, and can be studied in a metabolic ward under conditions where 'cheating' is virtually impossible without being detected. Usually these are middle-aged women who have been perhaps 40 kg overweight, and who have already lost about 20 kg. They are often depressed, hypothermic, and have a low metabolic rate. The nature of this metabolic adaptation to a low calorie diet is not known (as of 1973), but it is a phenomenon which has been known since before 1920. [1](J S Garrow, 1973)
♦For obese individuals, a certain amount of weight loss is possible through a range of treatments, but long-term sustainability of lost weight is much more challenging, and in most cases, the weight is regained [2]. In a meta-analysis of 29 long-term weight loss studies, more than half of the lost weight was regained within two years, and by five years, more than 80% of lost weight was regained [3,4].
In addition, studies of those who are successful at sustained weight loss indicate that the maintenance of a reduced degree of body fatness will probably require close attention to energy intake and expenditure, perhaps for life[5].
<Metabolic values of obese individuals>
♦The hypometabolic thesis had fallen out of favor by 1930, when more accurate calculations of body-surface area indicated that the metabolic rates of obese individuals were normal[6].
♦Total energy expenditure (TEE) in a day consists of three components: diet-induced thermogenesis (DIT), physical activity energy expenditure (PAEE), and resting energy expenditure (REE). When comparing a model case of men with an average weight of 100 kg and 70 kg, the man weighing 100 kg has a higher TEE[7] .
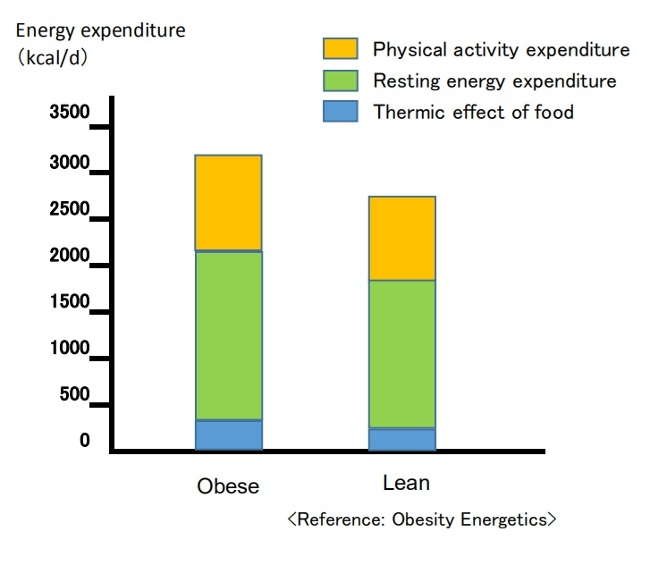
(Breakdown of energy expenditure in average 100-kg and 70-kg men)
Contrary to popular belief, people with obesity generally have a higher absolute REE compared to leaner subjects.This is because obesity increases both body fat and metabolically active fat-free mass[7,8].
PAEE can be subdivided into "voluntary exercise" and “activities of daily living.” Despite typically engaging in less physical activity, obese individuals often have a daily energy cost for physical activity similar to that of non-obese individuals since PAEE is proportional to body weight [7,9]. Additionally, due to a greater food intake, their DIT also tends to be higher [7].
<Dynamic changes in energy expenditure>
♦Obesity prevention is often erroneously described as a simple bookkeeping matter of balancing caloric intake and expenditure [10].
In this model, energy intake and expenditure are considered independent parameters determined solely by behavior. It is assumed that an obese person can steadily lose weight by eating less and/or moving more at a rate of one pound for every 3,500 kcal (or one kg for every 7,200 kcal) of accumulated dietary caloric deficit [7,11]. This view has been referred to as a “static model” of weight-loss, but it has been shown to be physiologically impossible [7,12].
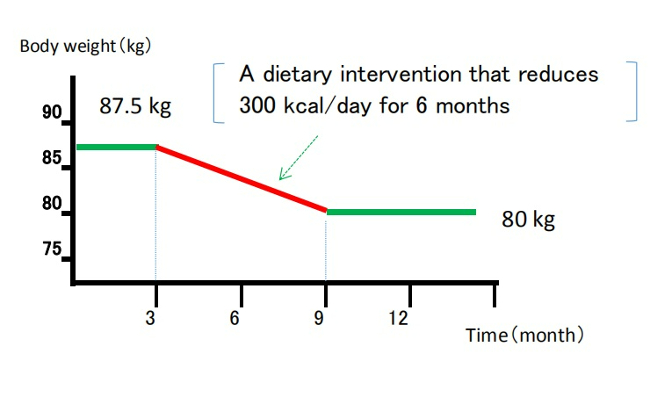
(Static model of weight loss)
(Despite being recognized as overly simplistic, the 3,500 kcal rule continues to appear in scientific literature and has been cited in over 35,000 educational weight-loss websites as of 2013.) [12,13]
♦It is now understood that energy intake and expenditure are interdependent variables, influenced by each other and by homeostatic signals triggered by changes in body weight [7,14].
Attempts to alter energy balance through diet or exercise are countered by physiological adaptations that resist weight loss [7].
<Set-point theory of body weight>
♦In recent years, the influence of homeostatic control has been recognized, and there is growing evidence that the body employs physiological mechanisms to manipulate energy balance and maintain body weight at a genetically and environmentally determined "set-point."[12]
In 1953, Kennedy proposed that body fat storage is regulated [15]. In 1982, nutritional researchers William Bennett and Joel Gurin expanded on Kennedy's concept when they developed the set-point theory [16]. The model has been widely adopted, and strengthened particularly after the discovery of leptin in the 1990’s [7,12].
When an individual loses weight, the body significantly reduces energy expenditure to a degree that is often greater than predicted based on changes in body composition and the thermic effect of food. It also causes an increase in appetite through hormonal regulation and alters food preferences through behavioral changes, to drive the body weight back toward its set-point range[7,16].
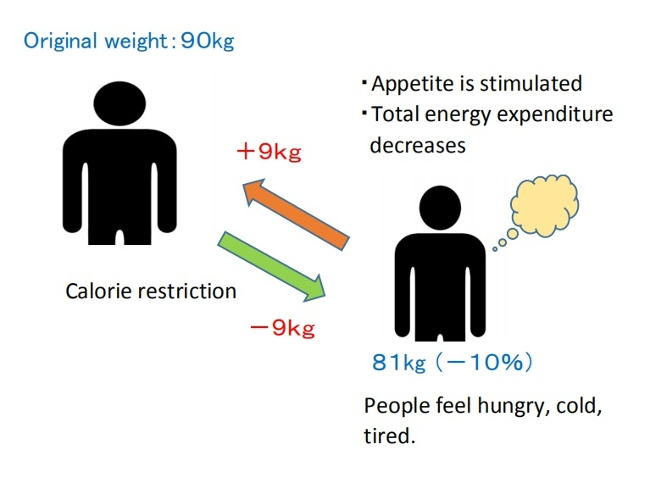
( Set-point model of weight loss)
♦Weight-loss studies have shown that the magnitude of fat stores in the body is protected by mechanisms mediated by the central nervous system, which adjust energy intake (EI) and expenditure (EE) via signals from adipose tissue, the gastrointestinal tract, and endocrine tissue to maintain homeostasis, and resist weight change (set-point model)[12,17].
♦The body's protective metabolic mechanism that attempts to preserve energy stores during an energy crisis is known as adaptive thermogenesis (AT) or metabolic adaption[7,12].
AT is defined as the underfeeding-associated fall in resting energy expenditure (REE), independent of changes in body composition[12].
♦Maintenance of a 10% or greater reduction in body weight in lean or obese individuals is accompanied by about 20 to 25% decline in 24-hour energy expenditure. This reduction in weight maintenance calories is 10 to 15% lower than what is predicted based solely on alterations (/changes) in fat and lean mass [17,18].
Since obese individuals also display these compensatory metabolic adjustments in response to dietary restriction, obesity may be considered a natural physiological state for some people. Experimental studies on obesity in animals similarly suggest a view of obesity as a condition of body energy regulation at an elevated set-point [19].
♦A meta-analysis of cross-sectional studies investigating adaptive thermogenesis (AT) by comparing formerly obese subjects who had lost weight with BMI-matched subjects who were never obese, reported a 3–5 % lower resting energy expenditure (REE) in formerly obese subjects compared to never obese controls[20].
This effect means, for example, that if an obese woman reduced her weight from 100kg to 70kg, she would have to consume fewer calories to remain at 70kg than a woman who had consistently weighed 70kg[6]. Similar results have been confirmed in animal experiments involving obese and normal-weight rats.
This suggests that the frequent claim made by obese people that they eat the same or less than their lean friends but lose no weight, must be given more credence than it is ordinarily accorded[19].
♦On the other hand, as shown in overfeeding experiments on prisoners in Vermont in the 1960’s (Doctor Ethan Sims), weight gain due to temporary overeating also triggers compensatory mechanisms that bring body weight back into a set-point range.
However, some researchers point out that these may be weaker than those protecting weight loss. This asymmetry could be due to the evolutionary advantage of storing fat to survive during periods of caloric restriction, such as prolonged starvation [16,17].

♦In addition, hyperphagia (overeating) has been demonstrated following experimental semi-starvation and short-term underfeeding, which is probably the result of homeostatic signals resulting from the loss of both body fat and lean tissue [7,21].
♦This theory also suggests that a person's set-point for body weight is established early in life and remains relatively stable unless altered by specific conditions. However, the set-point may change throughout one’s life due to factors such as marriage, childbirth, menopause, aging, and disease [16].
On the other hand, the set-point theory remains a theory because all the molecular mechanisms involved in set-point regulation have not been elucidated, and some researchers may consider this theory to be overly simplistic[16].
2. Problems with the set-point model
However, some researchers have pointed out problems with the set-point model of regulation.
If such a powerful biological feedback system regulating the state of our body fat exists, then why do many individuals in most Western countries gain weight throughout majority of their lives? In particular, they argue that this model cannot explain the increasing prevalence of obesity that has been observed in many societies worldwide since the 1970’s[22].
In response, some researchers argue that while metabolic resistance to sustaining a reduced body weight is strong, metabolic resistance to sustained increased adiposity may not be physiologically long-lasting. The steadily increasing prevalence of obesity in humans also suggests that the state of body fat is facilitated more vigorously than losing weight[17,23].
Animal studies in mice demonstrated increased energy expenditure and increased sympathetic nervous system (SNS) tone during the first 3-4 weeks of consuming a high-fat diet, while these changes were no longer evident after a few months of high-fat diet consumption[17,24].
Another animal study in mice reported that long-term consumption of palatable, high-energy diets such as potato chips, cheese crackers, cookies, etc., caused irreversible weight gain, suggesting an increase in the set-point weight[19,25]. This effect is believed to be due to the increases in the number of fat cell[19,26].

■These explanations may sound reasonable at first, but in my opinion, this is no longer a "set-point" and it fails to explain why the body stubbornly shows metabolic resistance to maintaining weight loss, even at a higher set-point weight.
When applied to humans, not everyone who frequently consumes high-calorie foods becomes obese. This brings up several contradictions, such as:
(1)Why is obesity more prevalent among the lower-income groups in Western societies[22,27] and relatively wealthier groups in developing societies?[28];
(2)The coexistence of undernutrition and obesity in poor populations, observed globally since the 1950’s[29];
(3) Why do some people gain weight after environmental changes such as entering college, getting married, having children, or moving from Asia to Western countries?[22]
As I have mentioned repeatedly in this blog, I believe that an increase in set-point for body weight is due to inducing intestinal starvation, and that I can explain all these contradictions.
In the following section, I will explain that more specifically.
3. Environmental and behavioral factors influencing weight set-point
In 2012, the American Association of Clinical Endocrinology (AACE) designated obesity as a chronic disease. One of the rationale for that designation is that, like other chronic diseases, the pathophysiology of obesity is complex, involving interactions of genetic, biological, environmental, and behavioral factors[30].
Some researchers interested in the body weight set-point theory argue that understanding how genes and the environment interact to regulate the set-point is essential to determining whether obesity, as a chronic disease, can be cured. However, they struggle to explain the many significant environmental and social influences[22].
■ I also believe that the set-point of body weight changes due to the interaction between (1) genetic and biological factors, and (2) environmental and behavioral factors, but this time, I will mainly discuss (2) in this article.
When considering changes in living environment since the 1970’s, most researchers blame the rise in obesity on the increased availability of high-calorie foods and a decrease in physical activity as society became more affluent. In other words, they believe that a positive energy balance is necessary for weight gain.
However, paradoxically, the fact that the increasing prevalence of obesity coincides with an increase in weight-loss attempts[31] suggests that our understanding of energy balance may be flawed[12].
As suggested by overfeeding experiments, “temporary weight gain from overeating” and “irreversible weight gain occurring over many years ” are different. In my view, obesity as a chronic disease arises rather from signals of negative energy balance or "food scarcity" in the body, as in the cases where people gain more weight than before after starvation or dieting.
[Related article]
The Overfeeding Study Suggests That "Overeating" Is Not the Cause of Obesity
■Despite its impact on our bodies due to changes in our living environments since the 1970’s, "intestinal starvation" remains unrecognized.
Intestinal starvation refers to a state where everything consumed is fully digested throughout the entire intestinal tract, and it can occur in today's affluent developed countries, developing countries, or even among the poor. When there is little or no fiber and everything has been digested, our bodies perceive it as having "no food."
【Related Article】
The Body Perceive That It Is More Starved than in the Past
Perhaps in many parts of the world before 1970, even if people went a whole day without eating until their next meal, there were likely still undigested substances like fiber and tough plant cell walls left in their intestines. However, in today’s society, with its abundance of easily digestible, refined carbohydrates, processed foods, and fast food, intestinal starvation can occur in as little as eight to ten hours, depending on eating habits.
Intestinal starvation is more likely to occur with frequent consumption of easily digestible refined carbohydrates (bread, noodles, rice, etc.), industrially processed meat or fish products, fast food, and snacks, etc., combined with a lack of vegetables and long periods of prolonged hunger (such as skipping breakfast, late-night meals, or irregular eating habits).
【Related Article】
■In the animal experiments on rats described in section 2 above, it was reported that consuming a "high-energy diet" for 90 days led to irreversible weight gain, suggesting an increase in the set-point. However, the "fattening” diet used in this experiment (Rolls et al., 1980) primarily consisted of highly palatable items like potato chips, cheese crackers, and cookies sold in supermarkets. Those foods contained 47.5% industrially processed refined carbohydrates (fat: 42%, protein: 10.5%) on an energy basis[25]. Moreover, rats only eat when they’re hungry, and they are capable of eating the same food repeatedly.
In contrast, the “chow” fed to the control rats may have been made from ingredients like ground wheat, ground corn, soybean meal, and fish meal, etc. In other words, just as our diets from over fifty years ago, it likely contained a high amount of indigestible components, such as fiber and tough plant cell walls.
Therefore, I question the conclusion that highly palatable "high-energy diets" alone caused the irreversible weight gain.
4. Why intestinal starvation increases one’s set-point weight
Below, I will explain the mechanism by which the set- point for body weight increases due to the induction of intestinal starvation. While some of it involves speculation, it is based on events that have happened to me several times. You may not believe it, but at least for me, it is 100% accurate.
■Let's assume there is a man who has maintained a weight of 70 kg for many years. Even though his weight may fluctuate slightly during busy periods or after overeating, his weight revolves around 70 kg, so his set-point would be considered 70 kg.
When intestinal starvation occurs, a signal that 'there is no food' is transmitted from the intestines (or small intestine) to the brain.
As a result, the body tries to absorb more nutrients, causing microscopic substances attached to the villi (or microvilli) in the small intestine to detach (Figure 1), thereby increasing the surface area for absorption and raising the absolute absorption ability.
In other words, I believe that weight gain, at least to some extent, involves not only an increase in body fat but also lean tissue such as muscle.
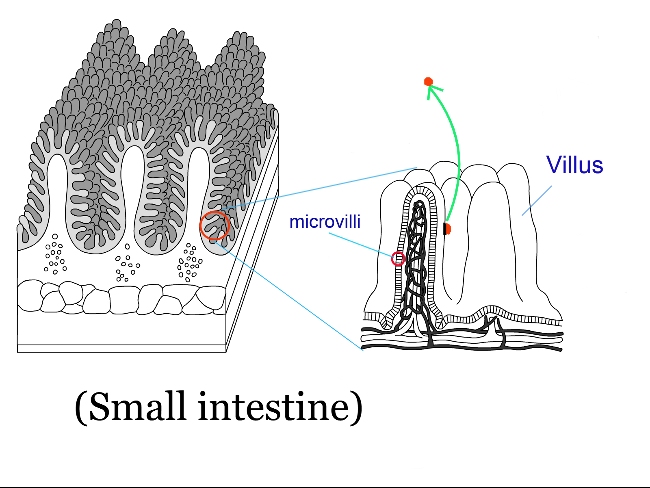
(Fig. 1 )
(Normally, a few undigested fiber or fats may remain, but when all food is perfectly digested, 5 to 10 kg of weight gain over a short period is possible.)
As a result, I believe the weight balancing point (the set-point) increases and reaches equilibrium within just a few days (Figure 2).
Weight gain does not occur from the gradual accumulation of excess calories each day, but rather in one sudden jump, possibly 0.3kg or 0.5g.
When dieting, the set-point can unknowingly rise, so you may gain a few pounds more than before once the diet ends.
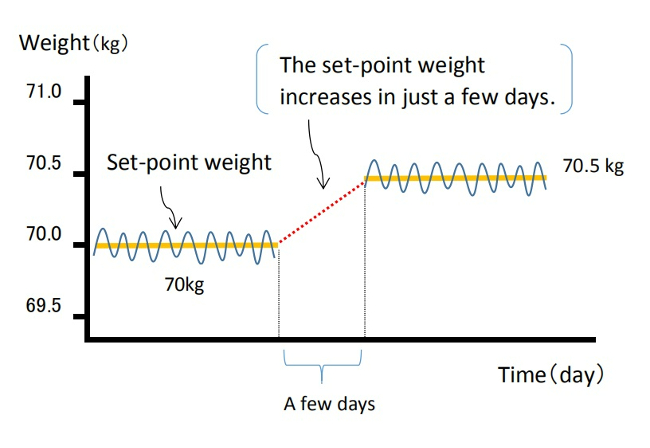
(Fig. 2 )
Once the body’s balance point rises, it becomes harder to lose weight because the overall absorption ability has increased. As mentioned in reference in section 1 , I agree with the idea that obesity is a “state of energy regulation at a higher set-point,” and is "a natural physiological condition" for obese individuals.
For more details, please refer to the article below.
【Related Article】
Gaining Weight by Intestinal Starvation; What Does It Mean?
<How to prove my theory>
It may be difficult to determine the exact cause when someone gains a few kg in a year, meaning their highest weight ever. However, I believe that, even with reduced calorie and carbohydrate intake from the meals I provide, it is possible to significantly increase a person's weight (by around 5 to 10 kg) within a few months, setting a new high set-point weight. By observing the data before and after, I think it is possible to investigate what changes have occurred inside the body.
<References>
[1]Garrow JS. Diet and obesity. Proc R Soc Med. 1973 Jul;66(7):642-4. PMID: 4741395; PMCID: PMC1645095.
[2]Wu T, Gao X, Chen M, van Dam RM. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev. 2009;10(3):313–323.
[3] Hall KD, Kahan S. Maintenance of Lost Weight and Long-Term Management of Obesity. Med Clin North Am. 2018 Jan;102(1):183-197.
[4]Anderson JW, Konz EC, Frederich RC, Wood CL. Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr. 2001 Nov;74(5):579-84.
[5]Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323-41.
[6]Jou C. The biology and genetics of obesity--a century of inquiries. N Engl J Med. 2014 May 15;370(20):1874-7.
[7]Hall KD, Guo J. Obesity Energetics: Body Weight Regulation and the Effects of Diet Composition. Gastroenterology. 2017 May;152(7):1718-1727.e3.
[8]Nelson KM, Weinsier RL, Long CL, et al. Prediction of resting energy expenditure from fat-free mass and fat mass. Am J Clin Nutr. 1992;56:848–856.
[9]Westerterp KR. Physical activity, food intake, and body weight regulation: insights from doubly labeled water studies. Nutr Rev. 2010;68:148–154.
[10] Levine DI. The curious history of the calorie in U.S. policy: a tradition of unfulfilled promises. Am J Prev Med. 2017;52:125–129.
[11] Hall KD, Chow CC. Why is the 3500 kcal per pound weight loss rule wrong? Int J Obes (Lond). 2013 Dec;37(12):1614.
[12] Egan AM, Collins AL. Dynamic changes in energy expenditure in response to underfeeding: a review. Proc Nutr Soc. 2022 May;81(2):199-212. doi: 10.1017/S0029665121003669. Epub 2021 Oct 4. PMID: 35103583.
[13]Thomas DM, Martin CK, Lettieri S et al. (2013) Can a weight loss of one pound a week be achieved with a 3500-kcal deficit? Commentary on a commonly accepted rule. In Int J Obes 37, 1611–1613.)
[14]Hall KD, Heymsfield SB, Kemnitz JW et al. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr. 2012 Apr;95(4):989-94.
[15]KENNEDY GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci. 1953 Jan 15;140(901):578-96.
[16] Ganipisetti VM, Bollimunta P. Obesity and Set-Point Theory. 2023 Apr 25. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 37276312.
[17] Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (Lond). 2010 Oct;34 Suppl 1(0 1):S47-55.
[18] Leibel R, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Eng J Med. 1995;332:621–28.
[19] Richard E. Keesey, Matt D. Hirvonen, Body Weight Set-Points: Determination and Adjustment, The Journal of Nutrition, Volume 127, Issue 9, 1997, Pages 1875S-1883S, ISSN 0022-3166.
[20]Astrup A, Gøtzsche PC, van de Werken K, et al. Meta-analysis of resting metabolic rate in formerly obese subjects. Am J Clin Nutr. 1999 Jun;69(6):1117-22.
[21] Dulloo AG, Jacquet J, Girardier L. Poststarvation hyperphagia and body fat overshooting in humans: a role for feedback signals from lean and fat tissues. Am J Clin Nutr. 1997;65:717–723.
[22]Speakman JR, Levitsky DA, Allison DB, et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis Model Mech. 2011 Nov;4(6):733-45.
[23] Schwartz MW, Woods SC, Seeley RJ, et al. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003 Feb;52(2):232-8.
[24] Corbett SW, Stern JS, Keesey RE. Energy expenditure in rats with diet-induced obesity. Am J Clin Nutr. 1986 Aug;44(2):173-80.
[25] Rolls B.J., Rowe E.A., Turner R.C. Persistent obesity in rats following a period of consumption of a mixed high energy diet. J Physiol. 1980 Jan;298:415-27.
[26]Faust I.M., Johnson P.R., Stern J.S., Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am. J. Physiol., 235 (1978), pp. E279-E286
[27] Dykes J et al. Socioeconomic gradient in body size and obesity among women: the role of dietary restraint, disinhibition and hunger in the Whitehall II study. International Journal of Obesity 2004 Feb,:262-68.
[28] Poskitt EM. Countries in transition: underweight to obesity non-stop? Ann Trop Paediatr. 2009 Mar;29(1):1-11.
[29] Gary Taubes. 2011. Why we get fat. New York: Anchor Books, Pages 31-40.
[30]Garvey WT. Is Obesity or Adiposity-Based Chronic Disease Curable: The Set Point Theory, the Environment, and Second-Generation Medications. Endocr Pract. 2022 Feb;28(2):214-222.
[31]Montani JP, Schutz Y, Dulloo AG. Dieting and weight cycling as risk factors for cardiometabolic diseases: who is really at risk? Obes Rev. 2015 Feb;16 Suppl 1:7-18.
06/20/2024
The Overfeeding Experiment Suggests That "Overeating" Is Not the Cause of Obesity
Contents
- Can overfeeding experiments make people obese?
- Subsequent overfeeding experiments
- Can metabolism explain this weight regain?
- Difference between obesity and overfeeding experiments: My thoughts
1. Can overfeeding experiments make people obese?
According to George A. Bray (as of 2020, an emeritus professor at Pennington Biomedical Research Center), until the 1960’s, obesity was viewed as a "lack of will power," and many people thought, and some said "if only these patients would push themselves away from the table, they would not have this problem."
With this view of obesity, he reflects that the turning point for obesity being accepted as a bona fide area of academic interest were the studies on overfeeding. Overfeeding studies began to provide valuable insights into the biology of obesity. For Doctor Bray, who was a postdoctoral fellow at the New England Medical Center Hospital in Boston at the time, the excitement that was generated when the Vermont overfeeding studies were first presented in 1968 was unforgettable[1].
This was the case with the overfeeding experiments conducted by Doctor Ethan Sims in the late 1960’s. Until then, it was commonly believed that, "overeating obviously leads to obesity," so few such experiments had been conducted.
According to Dr. Jason Fung, the author of “The Obesity Code,” Dr. Sims recruited lean students at the nearby University of Vermont and encouraged them to eat a lot to gain weight. However, despite what both he and the students had expected, the students did not become obese.
Suspecting that the students might have been increasing their exercise, Dr. Sims changed course. He then recruited convicts at the Vermont State Prison as subjects. Physical activity was strictly controlled, and attendants were present at every meal to ensure the calories-4000 per day-were eaten.
Although the prisoners’ weight initially rose, it then stabilized. While some prisoners gained more than twenty percent of their original body weight, the extent of weight gain varied significantly among them[2] .

Over two hundred days on this overfeeding regimen, twenty inmates gained an average of twenty to twenty-five pounds. (About 10 kg.) However, once the experiment ended and their caloric intake returned to normal, the men had difficulty maintaining the weight gain, and most shed all the weight they had gained relatively easily. The exceptions were two inmates who struggled to lose that weight[3].
At the time, Dr. Bray was allowed to participate as a co-researcher in Dr. Sims' experiment to examine the metabolic changes occurring in adipose tissue during the weight gain that followed overfeeding.
Later, in 1972, he conducted his own overeating experiment, using himself as a guinea pig. Initially, he tried to double what he usually ate at each meal, but he couldn't finish, so he switched to energy-dense foods, such as ice cream.
Over the next ten weeks, he gradually gained ten kilograms, and repeated his tests such as measuring the thermic effect of foods.
But once he stopped stuffing himself, his weight rapidly decreased, returning to his original seventy-five kilograms six weeks later, and he has maintained this weight with no trouble ever since.
Following his self-experiment, the other four volunteers in this study began overeating in the summer of 1972. When the experiment ended, all the volunteers returned to their baseline weight.

Dr.Bray states that this rapid return to normal weight contrasts with the difficulties people with spontaneous obesity have in losing weight, and the even more difficult task of a maintaining a lower weight. Many who develop obesity over years suffer from a different kind of pain than those of us who acutely gain weight by overfeeding. For them, the obesity “slips up” on them and once present, is difficult to reverse.
The history of overfeeding and underfeeding trials and other lines of evidence clearly show that obesity prevention and treatment cannot simply rely on the advice to "eat less and exercise more." [1]
2. Subsequent overfeeding experiments
Alex Leaf (Western States University) and Jose Antonio (Nova Southeastern University) reviewed overfeeding studies conducted up to 2017 that evaluated various combinations of macronutrient overfeeding and its effects on body composition.
They found twenty-five overfeeding studies that reported changes in fat mass (FM) and fat-free mass (FFM), in addition to changes in body weight. The study durations ranged from nine to one hundred days, and all but four were conducted in sedentary populations[4]. Notably, the objectives of each study varied, and not all mentioned weight loss following the end of the experiments.
To give a few examples, a study on identical twins was published in 1990.
■Bouchard (Laval University, Canada) et. al. recruited twelve pairs of young adult male identical twins (twenty-four individuals) with no exercise habits. Each participant's energy requirements were measured during a two-week base-line period, and after that, they were overfed by 1000 kcal per day (comprising 15% protein, 35% fat, and 50% carbohydrates), six days per week, for a total of eighty-four days during a one hundred-day period. The men were housed in a closed section of a university dormitory, and were under supervision by staff all day.

The mean weight gain was 8.1 kg, of which 67% was fat mass (FM). However, the weight gain varied widely among participants, ranging from 4.3 to 13.3 kg[5].
Four months after the experiment ended, the twins' average weight was 61.7 kg, which was only 1.3 kg higher than their baseline weight of 60.4 kg, indicating they had almost returned to their original weight[1].
■Conford (University of Michigan) et. al. conducted a study in 2012 involving nine healthy, non-obese adults (seven men and two women). The participants were admitted to the hospital for two weeks, during which time they ate 4000 kcals per day (comprising 15% protein, 35% fat, and 50% carbohydrates). Their energy requirements were determined during a one-week baseline period before the start of the experiment. In addition to three main meals, they had four snacks each day. The average weight gain was 2.1 kg, of which 67 % was fat mass (FM).[6]
The summary of this review indicates that overfeeding healthy, sedentary adults with a diet moderately high in both carbohydrates and fats (35-50% energy intake each) and low in protein (11-15%) primarily results in a gain in fat mass (FM), which accounts for 60-70 % of the weight gain. Additionally, the increase in fat-free mass (FFM) may be due to an increase in body water content rather than skeletal muscle tissue. In contrast, diets with significantly increased protein intake showed favorable changes in body composition, even with increased energy intake[4].
3. Can metabolism explain this weight regain?
Why did the participant’s weight rapidly return to normal over the ensuing weeks when they stopped overeating?
According to Dr. Bray, one of the striking findings in this Vermont study was that to maintain the weight they gained after overfeeding, they required more energy per unit surface area than before weight gain. When Dr. Bray moved to the University of California in 1970, his new lab began operating to explore his hypothesis about why extra energy was required to maintain the increased weight[1].
■Leibel (Rockefeller University) et. al. conducted a study in 1995 involving eighteen obese (BMI of 28 or higher) subjects (Group A) and twenty-three subjects who had never been obese (Group B).
They measured changes in energy expenditure under three conditions: at their usual body weight, after losing more than 10 percent of their body weight by underfeeding, and after gaining 10 percent of their body weight by overfeeding.
When maintaining a body weight at a level 10% or more below their initial weight, the total energy expenditure decreased by 8±5 kcal per kilogram per day in Group A and by 6±3 kcal in Group B.
Conversely, when maintaining a body weight at a level 10% above their initial weight, total energy expenditure increased by 8±4 kcal in Group A and by 9±7 kcal in Group B.
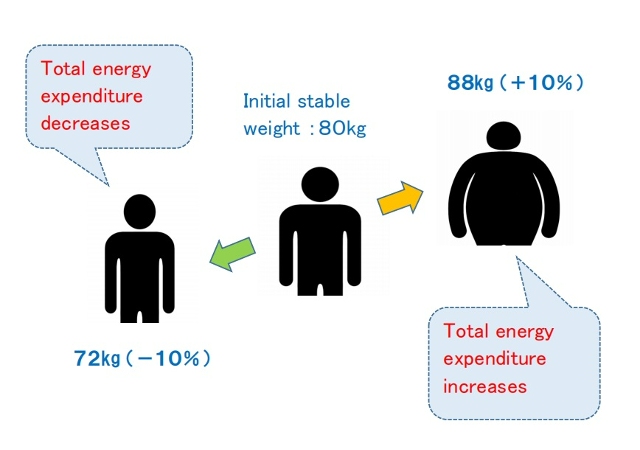
The study concluded that maintenance of a reduced weight or elevated body weight is associated with compensatory changes in energy expenditure, which resist maintaining the altered body weight and function to restore the original weight. This suggests that the long-term effectiveness of obesity treatment through caloric reduction may be limited[7].
4. Difference between obesity and overfeeding experiments: My thoughts
As Dr. Bray mentioned, I also believe that weight gain from temporary excessive caloric intake is due to body mechanisms entirely different from those underlying fundamental obesity. If compensatory metabolic mechanisms resist changes in body weight, why do some people continue to gain weight?
As I have repeatedly mentioned through this blog, the difference between people who are overweight and lean can be explained by the difference in set-point for body weight. (One's set-point weight goes up through intestinal starvation.)
For example, suppose a person who normally weighs a stable sixty kg is temporarily overfed and reaches sixty-three kg.
This can be compared to a glass of water that is usually filled to about 97% now being filled to 100%, and then surface tension causing the water to rise above the rim.
Conversely, maintaining a weight of fifty-six kg by eating less is like the water level temporarily decreasing, causing a dip in the surface of the water.
In both cases, the set-point weight has not changed.
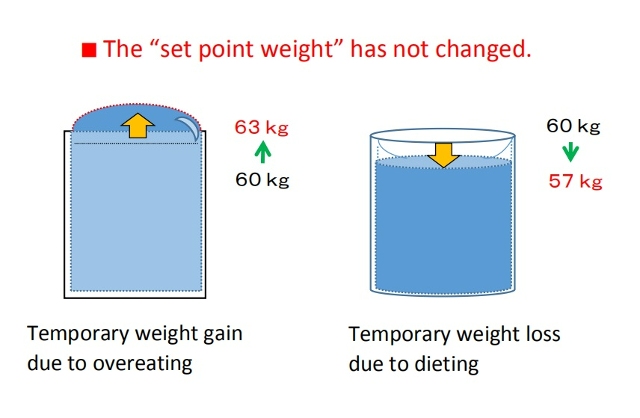
In contrast, if a person who originally weighs sixty kg gradually gains weight over several years, and then maintains a stable weight of ninety kg, this indicates that the set-point weight itself has increased, meaning the glass has grown larger, with energy balancing at a higher level.
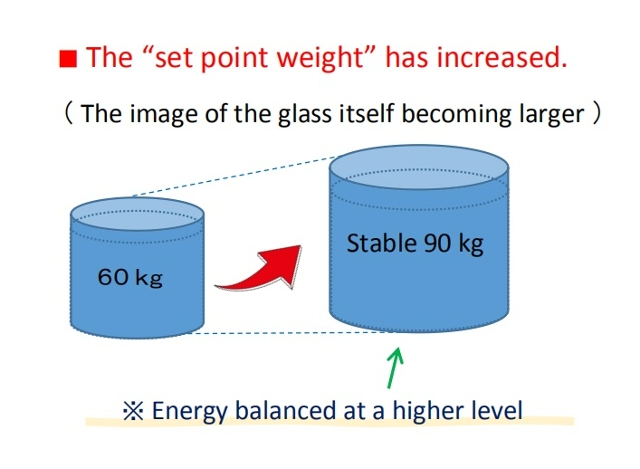
Today, we are sometimes said to be living in an "obesogenic environment" that promotes obesity, but that does not necessarily mean consuming high-calorie foods or living sedentary lifestyles. As some researchers have already mentioned, calorie counting is clearly of little significance. Changes in caloric intake only lead to temporary weight gain or loss.
Rader, the "obesogenic environment" in my opinion is related to foods that are overly digestible foods (refined carbohydrates, fast food, processed foods, etc.) and an imbalanced diet (lack of vegetables, etc.). When these factors overlap with some other conditions such as skipping breakfast or eating late dinners, intestinal starvation is more likely to be induced.
Of course, researchers will say that in order to gain weight, more energy must be taken into the body than before. For more on why intestinal starvation leads to greater energy intake and weight gain, please refer to the article below.
[Related article] Gaining Weight by Intestinal Starvation; What Does It Mean?
<References>
[1]Bray GA. The pain of weight gain: self-experimentation with overfeeding. Am J Clin Nutr. 2020 Jan 1;111(1):17-20.
[2] Fung J, The Obesity Code, Greystone books, 2016, P114-116.
[3] Jou C. The biology and genetics of obesity--a century of inquiries. N Engl J Med. 2014 May 15;370(20):1874-7.
[4] Leaf A, Antonio J. The Effects of Overfeeding on Body Composition: The Role of Macronutrient Composition. Int J Exerc Sci. 2017 Dec 1;10(8):1275-1296.
[5] Bouchard C et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990 May 24;322(21):1477-82.
[6] Cornford AS et al. Rapid development of systemic insulin resistance with overeating is not accompanied by robust changes in skeletal muscle glucose and lipid metabolism. Appl Physiol Nutr Metab. 2013 May;38(5):512-9.
[7] Leibel RL et al. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995 Mar 9;332(10):621-8.
- 1 / 9
- »

